The Leading Source of Diabetes Business News the Long View Fall
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
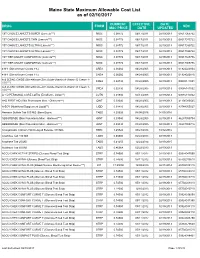
Maine State Maximum Allowable Cost List As of 02/10/2017
Maine State Maximum Allowable Cost List as of 02/10/2017 CURRENT EFFECTIVE DATE DRUG FORM NDC MAC PRICE DATE UPDATED 1ST CHOICE LANCETS SUPER (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517065722 1ST CHOICE LANCETS THIN (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517015722 1ST CHOICE LANCETS ULTRA (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517030722 1ST CHOICE LANCETS ULTRA (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517035722 1ST TIER UNILET COMFORTOU (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517025736 1ST TIER UNILET COMFORTOU (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517065736 4-N-1 (Dimethicone Cream 1%) CREA 0.06060 04/28/2005 02/19/2013 61924020804 4-N-1 (Dimethicone Cream 1%) CREA 0.06060 04/28/2005 02/19/2013 61924020816 A & D ZINC OXIDE (Dimethicone-Zinc Oxide-Vitamin A-Vitamin D Cream 1- CREA 0.02730 04/28/2005 02/19/2013 00085141001 10%***) A & D ZINC OXIDE (Dimethicone-Zinc Oxide-Vitamin A-Vitamin D Cream 1- CREA 0.02730 04/28/2005 02/19/2013 00085141002 10%***) A + D PERSONAL CARE LOTIO (Emollient - Lotion**) LOTN 0.01940 03/13/2008 02/19/2013 00085113002 A+D FIRST AID (Skin Protectants Misc - Ointment***) OINT 0.03040 04/28/2005 02/19/2013 41100080545 A-SOY (Nutritional Supplement Liquid**) LIQD 0.01410 04/28/2005 02/19/2013 83744055027 Abacavir Sulfate Tab 300 MG (Base Equiv) TABS 4.20626 04/08/2016 04/05/2016 ABSORBASE (Skin Protectants Misc - Ointment***) OINT 0.03040 04/28/2005 02/19/2013 46287050704 ABSORBASE (Skin Protectants Misc - Ointment***) OINT 0.03040 04/28/2005 02/19/2013 -

“The Silver Sheet” MEDICAL DEVICE QUALITY CONTROL
F-D-C REPORTS — FOUNDED 1939 $665 A YEAR ® www.TheSilverSheet.com“The Silver Sheet” MEDICAL DEVICE QUALITY CONTROL Vol. 13, No. 2 February 2009 THE NEWS THIS ISSUE • RECALLS SOARED TO THEIR HIGHEST POINT EVER IN 2008, with companies recalling a total of 845 devices. Many of the recalls were related to software problems and tainted heparin. “The goal is to try and decrease those recalls,” says Tim Ulatowski, director of CDRH’s Office of Compliance. “So we’re trying to push that number down through effective enforcement actions and industry training.” There is good news, however: The number of Class I recalls fell from 23 in 2007 to only 17 in 2008 – industry’s best Class I showing since 2003. Also, the recall manager for a Virginia hospital chain tells what companies should do to make their recall letters clearer and easier to comply with. “We get some recall letters and we truly don’t know what to do with them,” Bea Haupt says................................................Below • COMPLETE TABLE OF RECALLS FROM 2008 includes 17 Class I (2%), 726 Class II (86%) and 107 Class III (12%) medical device events ............................................................................................................12 • WARNING LETTERS: I-Flow cited for QS and MDR reg violations in relation to its infusion pumps.........66 • IN BRIEF: New GHTF guidance instructs firms on the control of products and services obtained from suppliers; former OSEL Director Larry Kessler is concerned that CDRH doesn’t always follow scientific principles and practices .......................................................................................................Back page Problems Related To Software, Heparin Help Push Recalls To All-Time High Also, FDA And Industry Experts Give Tips For Composing Recall Letters The number of recalled medical devices counting methods are typically negligible; the skyrocketed last year to its highest point ever, agency counted 831 total recalls in FY 2008 and spurred on by a large volume of Class II recalls 664 in FY 2007. -

SWHPCGV (A6) Scott & White Health Plan Group Value Closed
SWHP Group Value Formulary Federal Employees Health Benefits Program 3rd Quarter 2021 P a g e | 2 Table of Contents What is my prescription drug coverage? ...................................................................... 3 What is the Scott & White Health Plan Group Value Formulary? .................................. 3 How was the formulary created and how are new medications reviewed? .................. 3 Does the formulary ever change? ................................................................................ 3 How am I notified of changes to the formulary? .......................................................... 4 What are brand-name and generic drugs? ................................................................... 4 What is generic substitution? ...................................................................................... 4 What are specialty drugs? ........................................................................................... 4 What are pharmaceutical management procedures?................................................... 5 Are there any restrictions on my coverage? ................................................................. 5 How do I request an exception to the SWHP formulary?.............................................. 5 What drugs are not covered by my prescription drug benefit? ..................................... 5 How much medication does my copayment cover and does my plan cover maintenance medications? ........................................................................................ -

2021 Bright Formulary (List of Covered Drugs)
2021 Bright Formulary (List of Covered Drugs) Bright Health Individual and Family Plans Colorado PLEASE READ: This document contains information about the drugs Bright Health covers in their Individual and Family plans. This formulary was updated on 09/01/2021. For more recent information or other questions, please contact us at 833-661-1988 or visit www.brighthealthplan.com. PA - Prior Authorization QL - Quantity Limits ST - Step Therapy OTC - Over the i counter Welcome to Bright Enclosed you will find a list of the drugs included in our Bright Health Individual and Family plans from January 1, 2021 - December 31, 2021. As you review, be sure to have your medications on hand so you can confirm your prescriptions are covered and compare dosage and pricing of the drugs you take. Keep in mind, this document includes a comprehensive list of drugs (formulary) included in our Individual and Family plans. For an updated formulary, please contact us. Our contact information, along with the date we last updated the formulary, appears on the front and back cover pages. As a Bright Health member, you must generally use in-network pharmacies to fill your prescriptions. Benefits, formulary, pharmacy network, and/or copayments/coinsurance may change on January 1, 2022, and from time to time during the 2021 calendar year. Sincerely, Your Bright Health team PA - Prior Authorization QL - Quantity Limits ST - Step Therapy OTC - Over the ii counter Frequently Asked Questions: What is a Formulary (drug list)? A formulary is a list of covered drugs selected by Bright Health in consultation with a team of health care providers, which represents the prescription therapies believed to be a necessary part of a quality treatment program. -

Wellcare of South Carolina Medicaid Preferred Drug List
2021 South Carolina Medicaid Comprehensive Preferred Drug List (List of Covered Drugs) Lista integral de medicamentos preferidos de South Carolina Medicaid (Lista de medicamentos cubiertos) WellCare of South Carolina 00 Please read: This document contains information about the drugs we cover in this plan. Please note: The South Carolina Medicaid Preferred Drug List is updated quarterly. Providers, please visit our website at https://www.wellcare.com/South- Carolina/Providers/Medicaid/Pharmacy to view updates to the preferred drug list. Members, please visit our website at https://www.wellcare.com/South-Carolina/Members/Medicaid-Plans/WellCare-of-South-Carolina/Pharmacy- Services to view updates to the preferred drug list. Importante: Este documento contiene información acerca de los medicamentos que tienen cobertura con este plan. Tenga en cuenta lo siguiente: La lista de medicamentos preferidos de South Carolina Medicaid se actualiza cada trimestre. Proveedores: visite nuestro sitio web en https://www.wellcare.com/South- Carolina/Providers/Medicaid/Pharmacy para ver las actualizaciones de la lista de medicamentos preferidos. Miembros: visite nuestro sitio web en https://www.wellcare.com/South-Carolina/Members/Medicaid-Plans/WellCare-of-South-Carolina/Pharmacy- Services para ver las actualizaciones de la lista de medicamentos preferidos. Last updated (4/1/2021) Última actualización (4/1/2021) CAD_67295M State Approved 01282021 ©WellCare 2021 SC1SMDCVR68980M_2021 Drug Name Preference Details Coverage Details *Adhd/Anti-Narcolepsy/Anti- -
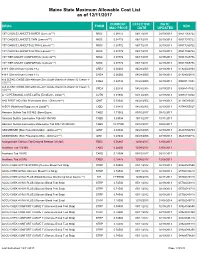
Mainecare MAC List Effective 12.11.2017
Maine State Maximum Allowable Cost List as of 12/11/2017 CURRENT EFFECTIVE DATE DRUG FORM NDC MAC PRICE DATE UPDATED 1ST CHOICE LANCETS SUPER (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517065722 1ST CHOICE LANCETS THIN (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517015722 1ST CHOICE LANCETS ULTRA (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517030722 1ST CHOICE LANCETS ULTRA (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517035722 1ST TIER UNILET COMFORTOU (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517025736 1ST TIER UNILET COMFORTOU (Lancets***) MISC 0.08772 06/17/2011 02/19/2013 08517065736 4-N-1 (Dimethicone Cream 1%) CREA 0.06060 04/28/2005 02/19/2013 61924020804 4-N-1 (Dimethicone Cream 1%) CREA 0.06060 04/28/2005 02/19/2013 61924020816 A & D ZINC OXIDE (Dimethicone-Zinc Oxide-Vitamin A-Vitamin D Cream 1- CREA 0.02730 04/28/2005 02/19/2013 00085141001 10%***) A & D ZINC OXIDE (Dimethicone-Zinc Oxide-Vitamin A-Vitamin D Cream 1- CREA 0.02730 04/28/2005 02/19/2013 00085141002 10%***) A + D PERSONAL CARE LOTIO (Emollient - Lotion**) LOTN 0.01940 03/13/2008 02/19/2013 00085113002 A+D FIRST AID (Skin Protectants Misc - Ointment***) OINT 0.03040 04/28/2005 02/19/2013 41100080545 A-SOY (Nutritional Supplement Liquid**) LIQD 0.01410 04/28/2005 02/19/2013 83744055027 Abacavir Sulfate Tab 300 MG (Base Equiv) TABS 1.71062 09/15/2017 09/12/2017 Abacavir Sulfate-Lamivudine Tab 600-300 MG TABS 5.09004 10/13/2017 10/11/2017 Abacavir Sulfate-Lamivudine-Zidovudine Tab 300-150-300 MG TABS 19.77500 03/10/2017 03/08/2017 -

Muraglitazar Bristol-Myers Squibb/Merck Daniella Barlocco
Muraglitazar Bristol-Myers Squibb/Merck Daniella Barlocco Address Originator Bristol-Myers Squibb Co University of Milan . Istituto di Chimica Farmaceutica e Tossicologica Viale Abruzzi 42 Licensee Merck & Co Inc 20131 Milano . Italy Status Pre-registration Email: [email protected] . Indications Metabolic disorder, Non-insulin-dependent Current Opinion in Investigational Drugs 2005 6(4): diabetes © The Thomson Corporation ISSN 1472-4472 . Actions Antihyperlipidemic agent, Hypoglycemic agent, Bristol-Myers Squibb and Merck & Co are co-developing Insulin sensitizer, PPARα agonist, PPARγ agonist muraglitazar, a dual peroxisome proliferator-activated receptor-α/γ . agonist, for the potential treatment of type 2 diabetes and other Synonym BMS-298585 metabolic disorders. In November 2004, approval was anticipated as early as mid-2005. Registry No: 331741-94-7 Introduction [579218], [579221], [579457], [579459]. PPARγ is expressed in Type 2 diabetes is a complex metabolic disorder that is adipose tissue, lower intestine and cells involved in characterized by hyperglycemia, insulin resistance and immunity. Activation of PPARγ regulates glucose and lipid defects in insulin secretion. The disease is associated with homeostasis, and triggers insulin sensitization [579216], older age, obesity, a family history of diabetes and physical [579218], [579458], [579461]. PPARδ is expressed inactivity. The prevalence of type 2 diabetes is increasing ubiquitously and has been found to be effective in rapidly, and the World Health Organization warns that, controlling dyslipidemia and cardiovascular diseases unless appropriate action is taken, the number of sufferers [579216]. PPARα agonists are used as potent hypolipidemic will double to over 350 million individuals by the year compounds, increasing plasma high-density lipoprotein 2030. Worryingly, it is estimated that only half of sufferers (HDL)-cholesterol and reducing free fatty acids, are diagnosed with the condition [www.who.int]. -

7-Azaindoles and Their Use As Ppar Agonists 7-Azaindole Und Ihre Verwendung Als Ppar-Agonisten 7-Azaindoles Et Leur Utilisation Comme Agonistes De Ppar
(19) TZZ___T (11) EP 1 794 159 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 471/04 (2006.01) A61K 31/437 (2006.01) 01.08.2012 Bulletin 2012/31 A61P 3/10 (2006.01) (21) Application number: 05778246.8 (86) International application number: PCT/EP2005/009269 (22) Date of filing: 27.08.2005 (87) International publication number: WO 2006/029699 (23.03.2006 Gazette 2006/12) (54) 7-AZAINDOLES AND THEIR USE AS PPAR AGONISTS 7-AZAINDOLE UND IHRE VERWENDUNG ALS PPAR-AGONISTEN 7-AZAINDOLES ET LEUR UTILISATION COMME AGONISTES DE PPAR (84) Designated Contracting States: • GLIEN, Maike AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 65195 Wiesbaden (DE) HU IE IS IT LI LT LU LV MC NL PL PT RO SE SI • SCHAEFER, Hans-Ludwig SK TR 65239 Hochheim (DE) • WENDLER, Wolfgang (30) Priority: 11.09.2004 EP 04021667 65618 Selters (DE) • BERNARDELLI, Patrick (43) Date of publication of application: F-78450 Villepreux (FR) 13.06.2007 Bulletin 2007/24 • TERRIER, Corinne F-93190 Livry Gargan (FR) (73) Proprietor: Sanofi-Aventis Deutschland GmbH • RONAN, Baptiste 65929 Frankfurt am Main (DE) F-92140 Clamart (FR) (72) Inventors: (56) References cited: • KEIL, Stefanie EP-A- 1 445 258 WO-A-2004/074284 65719 Hofheim (DE) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. -

The Top 30 Global Medical Device Companies
The Top 30 Global Medical Device Companies Gains Top Declines The success of the medical device industry is never more apparent than when you examine the numbers being produced by manufacturers in this market. Year by year, today’s top companies show that dedication to the bottom line can help amass steady growth, with most of the top 30 companies posting healthy double- digit gains over the prior fiscal year. Of course, with all the competition and consolidation occurring in daily business, some companies showed signs of weakness with flat or, even worse, declining sales and profits—however, they are in the minority. While US companies tend to dominate the top 30, the proliferation of international giants shows that the industry is generating more and more profits from global outreach efforts, particularly in China, Japan and Europe. The dollar’s fluctuating worth will surely continue to impact sales over time, though. As the following in-depth company examinations show, new product innovation usually plays the most prominent role in determining success and sustainability year after year. At least one of the companies on this list will disappear in 2006. Guidant will probably go down in history as one of the top newsmakers of 2005 due to Boston Scientific’s aggressive acquisition of the cardiovascular product maker—not to mention Guidant’s mounting legal troubles stemming from a slew of product recalls and reports of improper handling of related problems with its ICDs and pacemakers. Parent company Kodak has also been weighing the merits of selling its longtime healthcare division and, by this time next year, that segment could be operating under another owner. -

S 26948: a New Specific Peroxisome Proliferator–Activated Receptor
ORIGINAL ARTICLE S 26948: a New Specific Peroxisome Proliferator–Activated Receptor ␥ Modulator With Potent Antidiabetes and Antiatherogenic Effects Maria Carmen Carmona,1 Katie Louche,1 Bruno Lefebvre,2 Antoine Pilon,3 Nathalie Hennuyer,3 Ve´ronique Audinot-Bouchez,4 Catherine Fievet,3 Ge´rard Torpier,3 Pierre Formstecher,2 Pierre Renard,5 Philippe Lefebvre,2 Catherine Dacquet,5 Bart Staels,3 Louis Casteilla,1 and Luc Pe´nicaud1 on behalf of the Consortium of the French Ministry of Research and Technology OBJECTIVE—Rosiglitazone displays powerful antidiabetes benefits but is associated with increased body weight and adipo- genesis. Keeping in mind the concept of selective peroxisome he peroxisome proliferator–activated receptors proliferator–activated receptor (PPAR)␥ modulator, the aim of this (PPARs) (1) are transcription factors belonging study was to characterize the properties of a new PPAR␥ ligand, S to the nuclear receptor transcription factor fam- 26948, with special attention in body-weight gain. Tily (1). Three isoforms, PPAR␣,-␦, and -␥, have RESEARCH DESIGN AND METHODS—We used transient been described to have tissue-specific patterns of expres- transfection and binding assays to characterized the binding sion and function—the latter being highly expressed in characteristics of S 26948 and GST pull-down experiments to adipocytes and macrophages among other cell types (2–5). investigate its pattern of coactivator recruitment compared with The role of PPAR␥ on adipocyte differentiation has been rosiglitazone. We also assessed its adipogenic capacity in vitro extensively studied in vitro and in vivo (2,3,6,7). Forced using the 3T3-F442A cell line and its in vivo effects in ob/ob mice ␥ (for antidiabetes and antiobesity properties), as well as the expression of PPAR in nonadipogenic cells is sufficient to homozygous human apolipoprotein E2 knockin mice (E2-KI) (for induce adipocyte differentiation on treatment with specific antiatherogenic capacity). -

Role of Dual PPAR Gamma and Alpha Agonists in Diabetes Mellitus
23 Role of Dual PPAR γ and α Agonists in Diabetes Mellitus—Have They Met a Road Block or They Are Dead? Mohd Ashraf Ganie, Abdul Hamid Zargar Abstract: There are three peroxisome proliferator-activated receptors (PPARs) subtypes which are commonly designated PPAR alpha, PPAR gamma and PPAR beta/delta. PPAR alpha activation increases high density lipoprotein (HDL) cholesterol synthesis, stimulates “reverse” cholesterol transport and reduces triglycerides. PPAR gamma activation results in insulin sensitization and antidiabetic action. Combined treatments with PPAR gamma and alpha agonists may potentially improve insulin resistance and alleviate atherogenic dyslipidemia, whereas PPAR delta properties may prevent the development of overweight which typically accompanies “pure” PPAR gamma ligands. The new generation of dual-action PPARs—the glitazars, which target PPAR-gamma and PPAR-alpha (like muraglitazar and tesaglitazar) were on deck in late-stage clinical trials for sometime and were considered effective in reducing cardiovascular risk, but their long-term clinical effects were unknown. Thus glitazars offered a hope of a new approach to diabetes care addressing not just glycemia, but dyslipidemia and other components of the metabolic syndrome, though the side effect profile remains unknown. No human data is available, and so it remains highly speculative. The glitazars and on the newly published results for muraglitazar and tesaglitazar. “The PPAR-alpha is a good target and is being developed to yield more potent drugs that work through PPAR-alpha, and at the same time, improve on the PPAR-gamma. Efforts is on to get the glucose lowering with few of the adverse effects. This thinking has met with problems as many clinical trials have been terminated due to dominant side effects. -

George Grunberger, M.D., F.A.C.P., F.A.C.E
GEORGE GRUNBERGER, M.D., F.A.C.P., F.A.C.E. Chairman, Grunberger Diabetes Institute Clinical Professor, Department of Internal Medicine Clinical Professor, Center for Molecular Medicine & Genetics Wayne State University School of Medicine Professor, Department of Internal Medicine Oakland University William Beaumont School of Medicine Visiting Professor, First Faculty of Medicine Charles University, Prague (Czech Republic) Phone: (248) 335-7740 43494 Woodward Ave, ste 208, Bloomfield Hills, MI 48302 Fax: (248) 338-7979 e-mail: [email protected] EDUCATION 1973 BA degree (Biochemistry), Columbia College, New York, NY 1977 MD degree, New York University School of Medicine, New York, NY TRAINING 1977-1980 Residency, Internal Medicine Case Western Reserve University University Hospitals of Cleveland, Cleveland, Ohio 1980-1983 Fellowship (clinical and basic research), Endocrinology and Metabolism, Diabetes Branch, National Institute of Arthritis, Diabetes, Digestive and Kidney Diseases National Institutes of Health, Bethesda, Maryland FACULTY APPOINTMENTS, Wayne State University, Detroit, Michigan 1986-1992 Associate Professor (tenured), Department of Internal Medicine Associate Professor (tenured), Department of Molecular Biology and Genetics 1992-2002 Professor (tenured), Department of Internal Medicine 1995-1996 Interim Chairman, Department of Internal Medicine 1992-1994 Professor (tenured), Department of Molecular Biology & Genetics 1994-present Professor (tenured), Center for Molecular Medicine & Genetics 1986-present Regular member