Federal Register/Vol. 85, No. 202/Monday, October 19, 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Twhe Registration Form 2013
TWHE REGISTRATION FORM 2013 Dr. Jimmy Adams Brenda Barrow Dean Associate Professor Lamar Institute of Technology Lamar Institute of Technology Staff Member Faculty Member 409 839 2014 409 880 8848 [email protected] [email protected] Lauri Arnold Rachel Benham Program Director Financial Aid Specialist Lamar Institute of Technology Lamar University Faculty Member Staff Member 409 839 2050 409 880 1817 [email protected] [email protected] Twila Baker Dr. Sherry Benoit Director, Compliance and RisK Associate Vice President Management Lamar University Lamar University Staff Member Staff Member 409 880 1817 409 880 8933 [email protected] [email protected] Valarie BlacK Virginia Barron Director of Student Organizations Administrative Associate Senior Lamar University Lamar University Staff Member Staff Member 409 880 8739 409 880 7204 [email protected] [email protected] TWHE REGISTRATION FORM 2013 Ashley Boone Elizabeth Chapman Coordinator, University Success Seminar Student Services Coordinator, TALH Lamar University Lamar University Staff Member Staff Member 409 880 7207 409 839 2993 [email protected] [email protected] Diann Brodnax Robin Clements Coordinator VPAA Administrative Assistant III Lamar University Lamar State College- Orange Staff Member Staff Member 409 880 7960 409 882 3336 [email protected] [email protected] Carly Broussard Lisa Cowart Coordinator of Recruitment Director, Continuing and WorKforce Lamar University Education Staff Member Lamar State College- Orange 409 880 1704 Staff Member [email protected] 409 882 3321 [email protected] Maggie Cano Director of Recruitment Suzonne CrocKett Lamar University Instructor Staff Member Lamar State College- Orange 409 880 8535 Faculty Member [email protected] 409 988 8083 [email protected] TWHE REGISTRATION FORM 2013 Dr. -

Place Last Name First Namecity State Age Sex Time 1 Braslavskiyigor
Place Last Name First NameCity State Age Sex Time 1 BraslavskiyIgor Kiev, USSR 30 M 2:18:12 2 Kurtis Doug Northville MI 38 M 2:18:37 3 Nasedkin Leonid Kiev, USSR 28 M 2:18:49 4 Ayala Carlos Boulder CO 26 M 2:19:20 5 Schmidt Rolf Roseville MN 24 M 2:20:20 6 Gurny Shawmoir Poland 26 M 2:20:33 7 Stahl Kjell-erik Sweden 44 M 2:21:29 8 Yara Robert CockeyvilleMD 35 M 2:22:37 9 Featherby Neil England 32 M 2:23:15 10 Corrin Jan England 36 M 2:23:52 11 Pelarske Jim St Cloud MN 39 M 2:25:14 12 Malkowski Pawel Poland 31 M 2:27:44 13 Billig Patrick Roseville MN 28 M 2:27:55 14 Smoot Todd Matietta GA 28 M 2:28:08 15 Schlau Robert Charleston SC 42 M 2:28:18 16 Khlynin AleksanderKiev, USSR 33 M 2:28:23 17 ZimmermanThomas St Cloud MN 33 M 2:28:31 18 Rivas Carlos Boulder CO 27 M 2:29:08 19 Lind Alan Denver CO 30 M 2:29:12 20 Park Larry Houston TX 27 M 2:29:18 21 Ramierz Juan Tempe AZ 25 M 2:30:16 22 Chattin Chris Boulder CO 26 M 2:30:50 23 Moreno Angel Boulder CO 23 M 2:31:05 24 Whetham Rob Stillwater MN 38 M 2:31:48 25 Jarrow Duluth MN 28 M 2:32:26 26 Fisher David WhitewaterWI 26 M 2:32:53 27 Cottrell Harry Inver GroveMN Heights 44 M 2:33:16 28 Niemi Gene MinneapolisMN 32 M 2:33:20 29 Welzel Jane Fort CollinsCO 35 F 2:33:25 30 NiederbergerWilliam Madison WI 25 M 2:34:23 31 Raunig Deborah Great Falls MO 34 F 2:34:34 32 Jewett Wayne St Louis ParkMN 31 M 2:34:49 33 Johnsen Vern Carlton MN 32 M 2:35:40 34 Bloch Gordon New York NY 29 F 2:35:48 35 Daum Dave ChesterfieldMO 37 M 2:37:09 36 Eisenrich Thomas Plymouth MN 39 M 2:37:15 37 Delaney John Wellesley MA -

List of RN/LPN Required to Complete Fingerprinting by June 30, 2021
List of RN/LPN Required to Complete Fingerprinting by June 30, 2021 RN 74156 ABAD CHARLES U RN 61837 ACASIO KYRA K RN 75958 ADVINCULA ROLAND D J RN 75271 ABAD GLORY GAY M RN 59937 ACCOUSTI NEAL O RN 42910 ADZUARA MARY JANE R RN 76449 ABALOS JUDY S RN 52918 ACEBO RUSSELL J RN 72683 AETO JUSTIN RN 38427 ABALOS TRICIA H M RN 43188 ACEVEDO JUNE D RN 86091 AFONG CLAREN C D RN 70657 ABANES JANE J RN 68208 ACIDERA NORIVIE S RN 72856 AGACID MARIE JOY H RN 46560 ABANIA JONATHAN R RN 59880 ACIO ROSARIO A D RN 78671 AGANOS DAWN Y A V RN 67772 ABARQUEZ BLESSILDA C RN 72528 ACKLIN JUSTIN P RN 85438 AGARAN NOBEMEN O RN 67707 ABATAYO LIEZL P RN 68066 ACOB FERLITA D RN 52928 AGARAN TINA K LPN 7498 ABBEY LORI J RN 35794 ACOBA BETH MARY BARN RN 33999 AGASID GLISERIA D RN 77337 ABE DAVID K RN 73632 ACOBA JULIA R LPN 18148 AGATON KRYSTLE LIAAN RN 57015 ABE JAYSON K RN 53744 ACOPAN MARK N RN 66375 AGBAYANI REYNANTE LPN 6111 ABE LORI R RN 55133 ACOSTA MARISSA R RN 58450 AGCAOILI ANNABEL RN 18300 ABE LYNN Y LPN 18682 ACOSTA MYLEEN G A RN 54002 AGCAOILI FLORENCE A RN 73517 ABE SUN-HWA K RN 69274 ACRE-PICKRELL ALEXAN RN 79169 AGDEPPA MARK J RN 84126 ABE TIFFANY K RN 45793 ACZON-ARMSTRONG MARI RN 41739 AGENA DALE S RN 69525 ABEE JENNIFER E RN 19550 ADAMS CAROLYN L RN 33363 AGLIAM MARILYN A RN 69992 ABEE KEVIN ANDREW RN 73979 ADAMS JENNIFER N RN 46748 AGLIBOT NANCY A RN 69993 ABELLADA MARINEL G RN 39164 ADAMS MARIA V RN 38303 AGLUGUB AURORA A RN 66502 ABELLANIDA STEPHANIE RN 52798 ADAMSKI MAUREEN RN 68068 AGNELLO TAI D RN 83403 ABERILLA JEREMY J RN 84737 ADDUCCI -

Donations to River City Chorale We Gratefully Acknowledge the People Whose Generous Gifts Have Helped Us to Continue Our Musical Productions
Our 40th Anniversary Concert Spring Richard Morrissey, Director Dean Mora, Accompanist Featuring River City Chamber Choir Special Guest Performance Cowan Fundamental School Singers (Friday only) Please turn off all electrical devices. Audio or video recording and flash photography are prohibited. PROGRAM ADVERTISING SPONSORS We appreciate the support of these advertisers and we encourage you to save this program and patronize them. Ameriprise Financial Services, Inc. Ameritas Investment Corp. Miss Bliss Skin Care Christelle Wellness S. Curtis Fine Jewelry CWT Vacations Joseph de Illy, Attorney Dentistry of East Sacramento Dignity Health Dunnigan Realtors Eye Site Vision Care Franklin’s Family Auto Care Edi Guidi, DDS Hair by Cricket Hudson Appliance Repair Lighthouse Printing & Graphics Jerilyn Paik, Attorney Madison Station Cafe Marty Peterson, Travel Agent Private Piano Instruction Brad Rasor, Financial Advisor Serenity Spa and Soul Yoga Ron Sims Construction Services Kris Tague, Band Instrument Repair Textures Salon Tim’s Music Linda J. Vail, CPA Carrie Wheeler, Chiropractor Michael Yee, State Farm Insurance MISSED SEEING YOUR AD? For a very nominal fee, your ad can appear in three programs and on our website www.rivercitychorale.org/Advertise or call 916-427-0684 Chorale Members Dean Mora, Accompanist Soprano Alto Pam Adcock Anne Adrian Judy Andersen Carol Ann Baltzell Patty Budding Christina Chappell Meg Burnett Beth DePuy Rosemarie Felver Pat Edmonds Pat Junod Carol Eikelmann Elaine Kaul Brenda Drumm Kidd Lynn Lively Susan Kim Beth Messina Kelley Lints Pam Mitchell Kathy Midgley Cheri Nelson Cathy Nickeson Marty Peterson Cheryl Podesta Michelle Telles Dorothy Ritter Sharlene St. Clair Bass Cheryl Trevor Joe Cavness Trisha Uhrhammer Joe de Illy Barbara Washington Jim Eckerman Lois Wright Ken Eikelmann Sara Zeigler Myron Jantzen Noel Zimmermann Paul Johnson Jerrold Jones Clyde Kidd Tenor John Krilanovich Marilee Keene Chuck Maxson Doug Leggett Greg Meyer Frank Montez Paul Peterson Stan Muther Jerry Powell Vera Refnes R. -

Baby Girl Names Registered in 2005
Baby Girl Names Registered in 2005 # Baby Girl Names # Baby Girl Names # Baby Girl Names 1 Aaila 1 Abrieanna 1 Adinna 1 Aaliya 1 Abriel 1 Adisa 18 Aaliyah 1 Abriella 3 Adison 2 Aamna 1 Abrielle 1 Adisyn 1 Aanya 1 Abual 1 Aditi 2 Aaralyn 1 Abuk 1 Adlee 1 Aarilynn 1 Abul 1 Adlyn 1 Aarna 2 Abygail 1 Adna 1 Aarushi 1 Abygale 1 Adnee 1 Aasiyah 3 Acacia 1 Adreanna 1 AAya 1 Acadia 5 Adria 2 Abagail 1 Achan 2 Adrian 1 Abagayle 1Achinta 7 Adriana 1 Abang 1 Acia-Paris 1 Adrianah 1 Abay 1 Acok 15 Adrianna 1 Abbagayle 6 Ada 3 Adrianne 1 Abbegael 1 Adalaina 1 Adriella 1 Abbegail 1 Adalia 1 Adriene 14 Abbey 1 Adanna 6 Adrienne 1 Abbeygail 1 Adara 1 Adryanna 1 Abbeygayle 1 Adauny 1 Adut 1 Abbic 1 Adaya 1 Adysen 5 Abbie 1 Addie 1 Aelwyn 1 Abbigael 17 Addison 1 Aerianna 9 Abbigail 1 Addison-Shae 1 Aeriel 1 Abbigale 2 Addisyn 1 Aeris 2 Abbigayle 2 Addyson 1 Aeryn 1 AbbiGrace 1 Adea 1 Aeva 1 Abbirdina 1 Adeeba 1 Afaf 29 Abby 1 Adeela 2 Afnaan 1 Abbygael 1 Adela 1 Afnan 2 Abbygail 1 Adelaide 1 Afton 2 Abbygale 1 Adelaine 1 Agam 1 Abeam 1 Adele 4 Aganetha 1 Abeeha 1 Adelena 1 Agar 1 Abeera 1 Adeli 3 Agatha 1 Abeg 2 Adelina 1 Agnes 2 Abegail 3 Adeline 1 Agouideit 1 Abey 4 Adelle 1 Aguaer 1 Abi 1 Adelola 1 Ahlam 2 Abigael 1 Adelynn 1 Ahmeena 113 Abigail 1 Adeng 1 Ahona 4 Abigale 1 Aderyn 1 Ahyoung 4 Abigayle 1 Adesayo 1 Aicha 1 Abighail 1 Adessa 3 Aida 1 Abinash 1 Adeye 5 Aidan 1 Aboul 1 Adhel 1 Aiden 1 Abrar 1 Adia 2 Aidyn 1 Abree 1 Adila 1 Aidynn 1 Abrianna 1 Adilliya 1 Aiesha Baby Girl Names Registered in 2005 Page 2 of 36 January, 2005 # Baby Girl Names -
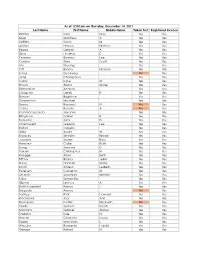
Last Name First Name Middle Name Taken Test Registered License
As of 12:00 am on Thursday, December 14, 2017 Last Name First Name Middle Name Taken Test Registered License Richter Sara May Yes Yes Silver Matthew A Yes Yes Griffiths Stacy M Yes Yes Archer Haylee Nichole Yes Yes Begay Delores A Yes Yes Gray Heather E Yes Yes Pearson Brianna Lee Yes Yes Conlon Tyler Scott Yes Yes Ma Shuang Yes Yes Ott Briana Nichole Yes Yes Liang Guopeng No Yes Jung Chang Gyo Yes Yes Carns Katie M Yes Yes Brooks Alana Marie Yes Yes Richardson Andrew Yes Yes Livingston Derek B Yes Yes Benson Brightstar Yes Yes Gowanlock Michael Yes Yes Denny Racheal N No Yes Crane Beverly A No Yes Paramo Saucedo Jovanny Yes Yes Bringham Darren R Yes Yes Torresdal Jack D Yes Yes Chenoweth Gregory Lee Yes Yes Bolton Isabella Yes Yes Miller Austin W Yes Yes Enriquez Jennifer Benise Yes Yes Jeplawy Joann Rose Yes Yes Harward Callie Ruth Yes Yes Saing Jasmine D Yes Yes Valasin Christopher N Yes Yes Roegge Alissa Beth Yes Yes Tiffany Briana Jekel Yes Yes Davis Hannah Marie Yes Yes Smith Amelia LesBeth Yes Yes Petersen Cameron M Yes Yes Chaplin Jeremiah Whittier Yes Yes Sabo Samantha Yes Yes Gipson Lindsey A Yes Yes Bath-Rosenfeld Robyn J Yes Yes Delgado Alonso No Yes Lackey Rick Howard Yes Yes Brockbank Taci Ann Yes Yes Thompson Kaitlyn Elizabeth No Yes Clarke Joshua Isaiah Yes Yes Montano Gabriel Alonzo Yes Yes England Kyle N Yes Yes Wiman Charlotte Louise Yes Yes Segay Marcinda L Yes Yes Wheeler Benjamin Harold Yes Yes George Robert N Yes Yes Wong Ann Jade Yes Yes Soder Adrienne B Yes Yes Bailey Lydia Noel Yes Yes Linner Tyler Dane Yes Yes -

Southern Utah State Co~E 1989 Saturday
Ni11e9'-Second Attttua! Commencement Southern Utah State Co~e 1989 Saturday. May 20. 7 p.m. Thunderbird Awards Night Commencement Auditorium Activities Thursday, May 25. II a.m. Academic Awards Convocation Auditorium Friday. fone 2. I p.m. White Ceremony fo r Nu rsing Graduates Adams Memorial 1 heatre Friday. June 2. 3:30-5:00 p.m. President's Reception Presidem·s Residence 331 West 200 South Friday, June 2, 7 p.m. Baccalaureate Services and Vocational Graduation Upper Campus Quadrangle Friday, June 2, 8:30 p.m. Baccalaureate Buffet (advance ticket purchase necessary) Great Hall Saturday. June 3, 9 a.m. Commencement Exercises Centrum Saturday, June 3, Noon Commencement Luncheon (advance ticket purchase necessary) Centrum South Lawn Saturday, June 3, 2 p.m. Rare Commissioning Ceremony Thorley Recital Hall Processional Baccafuureate Services Coronation March from The Prophet by Giacomo Meyerbeer arul Vocationa[ Graduation SUSC Orchestra Dr. Virginia Stitt. conducting Invocation Howard Lee Chamberiain Graduate, Class of 1989 Welcome President Gerald R. Sherratt Presentation of Distinguished Service Awards President Gerald R. Sherratt Citations Read by Members, SUSC Institutional Council Outstanding Student Address Amy Esplin Musical Number Rakoczy March By Franz Liszt SUSC Orchestra Dr. Virgina Stitt, conducting Baccalaureate Address Lee Roderick Washington Bureau Chief Scripps League Newspapers Awarding of Degrees and Certificates President Gerald R. Sherratt Presentation of Associate Degree and Vocational Certificate Recipients by Department Heads Benediction Sheri Lynne Brown Graduate, Class of 1989 Processional Commencement Arrival Traditional. Arranged by C. David Nyman E;t:ercises Barren Rocks of Aden Traditional, Arranged by C. David Nyman The Scarlet and Black Ceremonial Band C. -

LEHMAN COLLEGE TAVITA LUCKHAI and LORRAINE HENDERSON BAAH EMMANUEL BA 079 ART HISTORY ART HIS-30 079 Count 1 BERNARD
LEHMAN COLLEGE TAVITA LUCKHAI and LORRAINE HENDERSON Office of the Registrar Graduation Office - Shuster 105 (718) 960-8607 UNDERGRADUATE AND GRADUATE CANDIDATES FOR SEPTEMBER 1, 2009 UNDERGRADUATE CANDIDATES As of April 6, 2009 Last date to file April 10, 2009 BACHELOR OF ARTS Last Name First Name Degree Code Major BAAH EMMANUEL BA 079 ART HISTORY ART HIS-30 079 Count 1 BERNARDEZ WUILMER BA 112 ART CMP IMG/BA PALMER KACHELLE BA 112 ART CMP IMG/BA 112 Count 2 PERRY NICHELE BA 114 ART PHOTOG/BA 114 Count 1 LEON ALEXANDRA BA 121 BIOLOGY (68-70 CR) RAMOS ANA BA 121 BIOLOGY (68-70 CR) 121 Count 2 FULTON SHANEE BA 140 BLACK STUDIES 140 Count 1 ALFONSO MABEL BA 230 ECONOMICS AQUINO ROSA BA 230 ECONOMICS CONTRERAS JOSE BA 230 ECONOMICS ELLIS WESLEY BA 230 ECONOMICS GARCIA SUSIE BA 230 ECONOMICS HARB MARIANELA BA 230 ECONOMICS MARTE MAGDIER BA 230 ECONOMICS PAYNE LEE BA 230 ECONOMICS PEREZ DIOSAMNIS BA 230 ECONOMICS RENTA JENNIFER BA 230 ECONOMICS SWABY CHRISTOPHER BA 230 ECONOMICS 230 Count 11 CROSLEN SIMON BA 231 ACCOUNTING IND/GOVT MAJETTE ANGELA BA 231 ACCOUNTING IND/GOVT 231 Count 2 WALLACE TANYA BA 233 ACCOUNTING ACC/BUS-46 233 Count 1 CUELLO PEDRO BA 249 ACCOUNTING (42 CR) MAMUD ABDUL LATIF BA 249 ACCOUNTING (42 CR) 249 Count 2 FOSTER WILFRED BA 550 HISTORY LAMENDOLA JAMES BA 550 HISTORY MARTINEZ FRANCISCO BA 550 HISTORY PANTAZOPOULOS ERENE BA 550 HISTORY Page 1 WALLACE ANISHA BA 550 HISTORY 550 Count 5 CARR ANGELA BA 245 HISTORY CHLDHD ED 245 Count 1 SUERO LOYDA BA 247 HISTORY CH ED/BIL 247 Count 1 ZARRILLI ELIZABETH BA 314 PUERTO RICAN -

Table 4: Full List of First Forenames Given, Scotland, 2016 (Final) In
Table 4: Full list of first forenames given, Scotland, 2017 (final) in alphabetical order NB: * indicates that, sadly, a baby who was given that first forename has since died. Number of Number of Boys' names NB Girls' names NB babies babies A-Jay 2 Aabha 1 Aadam 4 Aadhya 1 Aaden 2 Aadhyaa 1 Aadhan 1 Aadia 1 Aadhith 1 Aadvika 1 Aadhrith 1 Aahliyah 1 Aadi 2 Aaida 1 Aadil 1 Aaila 1 Aadirath 1 Aailah 1 Aadrith 1 Aairah 5 Aahil 3 Aaishah 1 Aakin 1 Aaiylah 1 Aamir 2 Aaiza 1 Aaraf 1 Aakifa 1 Aaran 7 Aalayah 1 * Aarav 1 Aaleen 1 Aarif 1 Aaleyah 1 Aariv 1 Aalia 1 Aariz 2 Aaliya 1 Aaron 236 * Aaliyah 25 Aarron 6 Aaliyah-Louise 1 Aarush 4 Aaliyah-Mae 1 Aarya 1 Aaliyah-Raven 1 Aaryan 6 Aaliyah-Rose 1 Aaryn 3 Aamaya 1 Aaryn-Paul 1 Aara 1 Aashir 1 Aaria 3 Aayan 6 Aariah 2 Aayden 1 Aariyah 2 Aayden-James 1 Aarla 1 Aayden-Jon 1 Aarna 1 Aayush 1 Aarya 1 Abbas 2 Aathera 1 Abbdelrahmane 1 Aava 1 Abdalla 1 Aayat 3 Abdallah 2 Aayla 2 Abdelkrim 1 Abbey 4 Abdelrahman 1 Abbey-Leigh 1 Abderrahman 1 Abbi 4 Abderraouf 1 Abbie 52 Abdilahi 1 Abbie-Lee 1 Abdimalik 1 Abbie-Leigh 1 Abdoulie 1 Abbiegale 1 Abdul 8 Abby 19 Abdul-Haadi 1 Abby-Lily 1 Abdul-Hadi 1 Abeera 1 Abdul-Muizz 1 Aberdeen 1 Abdulfattah 1 Abi 7 Abduljabaar 1 Abigail 93 Abdullah 12 Abiha 3 Abdulmohsen 1 Abii 1 Abdulrahman 2 Abony 1 Abdulshakur 1 Abrish 1 Abdur-Rahman 2 Accalia 1 Abdurahman 1 Ada 33 Abdurrahman 1 Ada-Ellen 1 © Crown Copyright 2018 Table 4 (continued) NB: * indicates that, sadly, a baby who was given that first forename has since died Number of Number of Boys' names NB Girls' names NB -

Philanthropy at Unc Pembroke 2015
PHILANTHROPY AT UNC PEMBROKE 2015 Entrepreneurship Incubator Opens | P. 14 CHANGING LIVES THROUGH EDUCATION CONTENTS Message from Chancellor Robin Gary Cummings ︱ 4 Message from Vice Chancellor Wendy Lowery ︱ 6 University News Highlight ︱ 8 Record Enrollment University News Highlight︱ 10 Groundbreaking of the Health Services Building University News Highlight ︱ 12 Ribbon-cutting of the Entrepreneurship Incubator University News Highlight ︱ 14 Connect NC Bond to Fund New School of Business Facility Philanthropic Accomplishments ︱ 16 Meet Our Donors ︱ 18 Lumbee Guaranty Bank Meet Our Donor ︱20 Adolphus “Lee” Turner Meet Our Donors ︱ 22 Dr. Waltz and Dr. Louise Maynor Meet Our Donors ︱ 24 Sammy Cox Meet Our Donors︱ 26 Dr. Marian Wooten, OT Johnson, Sharlene Locklear Donors ︱ 28 Advancement Staff ︱ 41 2 | UNIVERSITY OF NORTH CAROLINA PEMBROKE UNC PEMBROKE ENTREPRENEURSHIP INCUBATOR UNIVERSITY OF NORTH CAROLINA PEMBROKE | 3 ince my earliest days, growing up in the shadow of Old Main, I’ve witnessed the immense pride in UNC Pembroke from people in the local community and far beyond. That pride is translated into generous financial support that benefits the university’s students and our Sability to reach off campus to serve the region. As Chancellor, I am committed to harnessing and growing an already strong spirit of generosity that surrounds UNCP. When I took office in July 2015, our advancement team had just marked the end of a fiscal year that saw more than $2 million in charitable giving, an increase of more than 18 percent over the previous year. There were many bright spots during the year, including growth in endowed scholarships, athletic and corporate giving, and alumni participation. -

Directory 2013
HAWAII STATE DEPARTMENT OF EDUCATION DIRECTORY 2013 The DOE Directory will be updated monthly. Updates will be posted at: doe.k12.hi.us or hawaiidoe.org October 2012 November 2012 December 2012 January/February 2013 FREQUENTLY CALLED NUMBERS A+ Program . 203-5510 Mathematics Education . 203-5537 Accounting . 586-3371 Migrant Education . 203-5520 Accreditation . 203-5570 Music Education . 203-5536 Adult Education. 203-5511 Para Educator . 586-4077 ext. 254 Advanced Placement . 203-5510 Payroll. 586-3181 Art Education . 203-5536 Personnel Regional Offices – Central. 627-7475 Athletics . 421-4394 Personnel Regional Offices – Hawaii . 974-6605 Background Check, Employees . 586-3607 Personnel Regional Offices – Honolulu . 733-4870 Board of Education . 586-3334 Personnel Regional Offices – Kauai. 274-3506 / 274-3507 Budget . 586-3350 Personnel Regional Offices – Leeward . 692-8007 Bus Transportation . 586-0170 Personnel Regional Offices – Maui . 984-8000 Career and Tech. Ed. 203-5532 Personnel Regional Offices – Windward . 233-5703 (ext. 277 or 276) Charter School Administrative Office . 586-3775 Physical Education . 203-5538 Child Abuse & Neglect Reporting Line . 832-5300 Pacific Resources for Education and Learning. 441-1300 Civil Rights Compliance Office . 586-3322 Printing (Reprographic Section) . 591-5500 Communications . 586-3230 Parent Teacher Student Association. 943-2042 Community Children’s Council Office . 586-5363 Project Inspire . 203-5510 Comprehensive Student Support Services Section. 205-5515 Public Charter Schools Program Office . 586-3570 Customer Service Desk . 564-6000 Public Schools of Hawaii Foundation . 943-1622 Drivers Education . 203-5510 Reclassification, Teacher . 586-3649 Early Childhood . 832-3303 Report Card . 203-5548 eCSSS, Help Desk . 564-6000 Response Center . -
2020 Delinquent Tax List (Pdf)
DELINQUENT TAX LIST OFFICE OF THE NYE COUNTY TREASURER AND EX-OFFICIO TAX RECEIVER As required by NRS 361.565 to be published, following are the names of property owners who are delinquent in the payment of taxes assessed against them for the taxable year beginning July 1, 2019 to June 30, 2020 775-482-8147 Parcel # Name Tax Penalties Mail/ Total Parcel # Name Tax Penalties Mail/ Total Amount Publishing Amount Publishing Cost Cost * Includes Prior Year Taxes * Includes Prior Year Taxes *** A *** 041-022-10 APOLLO DEVELOPMENT LLC $130.63 $22.30 $6.95 $159.88 036-471-03 A C E DEVELOPMENT ASSOCIAT $2,106.05 $404.55 $6.95 $2,517.55 035-721-01 APOLLO DEVELOPMENTS LLC $94.64 $20.82 $6.95 $122.41 045-131-10 A C E DEVELOPMENT ASSOCIAT $758.50 $153.33 $6.95 $918.78 035-721-02 APOLLO DEVELOPMENTS LLC $94.64 $20.82 $6.95 $122.41 045-131-16 A C E DEVELOPMENT ASSOCIAT $377.03 $81.79 $6.95 $465.77 035-721-03 APOLLO DEVELOPMENTS LLC $94.64 $20.82 $6.95 $122.41 040-662-10 AAQUIST, TONI LYNN $130.63 $22.30 $6.95 $159.88 035-721-04 APOLLO DEVELOPMENTS LLC $94.64 $20.82 $6.95 $122.41 042-062-11 AAQUIST, TONI LYNN $87.32 $19.21 $6.95 $113.48 035-721-05 APOLLO DEVELOPMENTS LLC $94.64 $20.82 $6.95 $122.41 036-061-47 ABANTO, SAMUEL E & LUCITA $103.26 $18.24 $6.95 $128.45 039-051-15 APOLLO DEVELOPMENTS LLC $196.63 $40.54 $6.95 $244.12 036-441-19 ABARQUEZ, SUSANA G $278.39 $96.63 $6.95 $381.97 039-701-01 APOLLO DEVELOPMENTS LLC $76.74 $16.88 $6.95 $100.57 036-441-20 ABARQUEZ, SUSANA G $278.39 $96.63 $6.95 $381.97 040-021-01 APOLLO DEVELOPMENTS LLC $85.71 $24.11