Synaptic Conductance Models
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Excitable Cell. Resting Membrane Potential and Action Potential (1&2)
The excitable cell. Resting Membrane Potential and Action Potential (1&2) Dr Sergey Kasparov School of Medical Sciences, Room E9 THIS TOPIC IS ALSO COVERED IN TUTORIALS! Teaching home page: http://www.bristol.ac.uk/phys-pharm/media/teaching/ Why do we call these cells “excitable”? Real action potentials.avi The key point: Bioelectricity is generated by the ions moving across cellular membranes Concentration of selected solutes in intracellular fluid and extracellular fluid in millimols mM 160 140 120 100 Insi de the cell 80 60 In extracellular fluid 40 20 0 1 The Na+/K+ pump outside inside [[mMmM]] + + [[mMmM]] + + + + + + + Na 145 + + 15 + K 4 + + 140 ATPATP ••isis an active ion transporter (ATP‐dependent) ••isis responsible for creating and upholding Na+ and K+ concentration gradients ••isis electrogenic – pumps 3 Na+ versus 2 K+ ions Reminder: Movement of ions is affected by concentration gradient and the electrical field Chemical driving force _ _ + + _ + + Electrical driving force _ + + _ + + + _+ _ + _ + + + + _ + _ + _ + + _ Net flux + + + + _ + _ + + V REMINDER: Diffusion through Ion Channels A leak channel A gated channel Both leak and gated channels allow movement of molecules (mainly inorganic ions) down the electrochemical gradient. So, if the gradient reverses, the ions will flow in the opposite direction. 1. The channels are aqueous pores through the membrane. 2. The channels are usually quite selective, for example some only pass Na+, others K+, still others – Cl- 3. Gated channels may be opened or closed by various factors -

Endogenous Serotonin Excites Striatal Cholinergic Interneurons Via The
Neuropsychopharmacology (2007) 32, 1840–1854 & 2007 Nature Publishing Group All rights reserved 0893-133X/07 $30.00 www.neuropsychopharmacology.org Endogenous Serotonin Excites Striatal Cholinergic Interneurons via the Activation of 5-HT 2C, 5-HT6, and 5-HT7 Serotonin Receptors: Implications for Extrapyramidal Side Effects of Serotonin Reuptake Inhibitors 1 1,2 3 1 3 2 Paola Bonsi , Dario Cuomo , Jun Ding , Giuseppe Sciamanna , Sasha Ulrich , Anne Tscherter , 1,2 3 ,1,2 Giorgio Bernardi , D James Surmeier and Antonio Pisani* 1 2 Fondazione Santa Lucia I.R.C.C.S., European Brain Research Institute, Rome, Italy; Clinica Neurologica, Dipartimento di Neuroscienze, 3 Universita` di Roma ‘Tor Vergata’, Rome, Italy; Department of Physiology, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA The striatum is richly innervated by serotonergic afferents from the raphe nucleus. We explored the effects of this input on striatal cholinergic interneurons from rat brain slices, by means of both conventional intracellular and whole-cell patch-clamp recordings. Bath- applied serotonin (5-HT, 3–300 mM), induced a dose-dependent membrane depolarization and increased the rate of spiking. This effect was mimicked by the 5-HT reuptake blockers citalopram and fluvoxamine. In voltage-clamped neurons, 5-HT induced an inward current, + + whose reversal potential was close to the K equilibrium potential. Accordingly, the involvement of K channels was confirmed either by + + increasing extracellular K concentration and by blockade of K channels with barium. Single-cell reverse transcriptase-polymerase chain reaction (RT-PCR) profiling demonstrated the presence of 5-HT2C, 5-HT6, and 5-HT7 receptor mRNAs in identified cholinergic interneurons. -

The Current-/Voltage-Clamp
SimNeuron Tutorial 1 SimNeuron Current-and Voltage-Clamp Experiments in Virtual Laboratories Tutorial Contents: 1. Lab Design 2. Basic CURRENT-CLAMP Experiments 2.1 Action Potential, Conductances and Currents. 2.2 Passive Responses and Local Potentials 2.3 The Effects of Stimulus Amplitude and Duration. 2.4 Induction of Action Potential Sequences 2.5 How to get a New Neuron? 3. Basic VOLTAGE-CLAMP Experiments 3.1 Capacitive and Voltage-dependent Currents 3.2 RC-Compensation 3.3 Inward and outward currents. 3.4 Changed command potentials. 3.5 I-V-Curves and “Reversal Potential” 3.6 Reversal Potentials 3.7 Tail Currents 4. The Neuron Editor 5. Pre-Settings 2 SimNeuron Tutorial 1. Lab Design We have kept the screen design as simple as possible for easy accessible voltage- and current-clamp experiments, not so much focusing on technical details of experimentation but on a thorough understanding of neuronal excitability in comparison of current-clamp and voltage-clamp recordings. The Current-Clamp lab and Voltage-Clamp lab are organized in the same way. On the left hand side, there is a selection box of the already available neurons with several control buttons for your recordings, including a schematic drawing the experimental set-up. The main part of the screen (on the right) is reserved for the diagrams where stimuli can be set and recordings will be displayed. Initially, you will be in the Current-Clamp mode where you will only see a current-pulse (in red) in the lower diagram while the upper diagram should be empty. Entering the Voltage-Clamp mode you will find a voltage-pulse in the upper diagram (in blue) with an empty lower diagram. -

Lecture 6 – Synaptic Transmission I -- Siegelbaum Postsynaptic
Lecture 6 – Synaptic Transmission I -- Siegelbaum Postsynaptic Mechanisms 1. Stretch receptor reflex as a model of neuronal signaling How does an impulse get transmitted from one cell to the next? 2. Two major forms of synaptic transmission a. Electrical – fast, bidirectional (usually), no amplification. Mediated by gap junctions. b. Chemical – slower, unidirectional, amplification. mediated by presynaptic terminals & postsynaptic receptors. 3. Gap junctions – consist of pairs of channels that span pre and postsynaptic membranes. protein subunits called connexin. Six subunits make one hemi-channel, called a connexon. 4. Chemical synapses – two major types of postsynaptic receptors a. Ionotropic receptors – receptor and ion channel in same macromolecule. Transmitter binding opens a channel. b. Metabotropic receptors – coupled to G proteins and second messengers. Indirectly influence ion channels. 5. Neuromuscular junction – Action potential in presynaptic terminals causes calcium influx via voltage- gated calcium channels Æ release of transmitter acetylcholine (ACh) Æ ACh diffusion across synaptic cleft to postsynaptic membrane Æ ACh binds to and opens nicotinic ACh receptor (nAChR), a ligand-gated ion channel Æ leads to Na+ influx into muscle cell Æ large, suprathreshold fast excitatory post-synaptic potential (EPSP) in postsynaptic muscle at end-plate. Reduce size of EPSP with curare so that it is subthreshold. See passive decay of EPSP amplitude away from endplate. 6. Nicotinic ACh receptor – a pentamer composed of four types of homologous subunits (two α subunits, and one β,γ and δ subunit). Each subunit contains a large external domain, four transmembrane domains, termed m1-m4, and a short external C terminus. Two molecules of ACh bind to the receptor. -

Serotonin and Cyclic Adenosine 3':Vmonophosphate Modulate The
0270-6474/85/0507-1862$02.00/0 The Journal of Neuroscience Copyright 0 Society for Neuroscience Vol. 5, No. 7, pi. 1862-1871 Printed in U.S.A. July 1985 Serotonin and Cyclic Adenosine 3’:VMonophosphate Modulate the Potassium Current in Tail Sensory Neurons in the Pleural Ganglion of ApJVsia ’ JONATHAN D. POLLOCK,’ LISE BERNIER,3 AND JOSEPH S. CAMARDO Department of Physiology, Center for Neurobiology and Behavior, College of Physicians and Surgeons, Columbia University and New York State Psychiatric Institute, New York, New York 10032 Abstract In sensory neurons of the abdominal ganglion of Aplysia califor- nica, which mediate the siphon-gill withdrawal reflex, serotonin elicits Tail sensory neurons in the pleural ganglion that mediate a slow, decreased conductance postsynaptic potential (slow PSP) the afferent portion of the tail withdrawal reflex in Ap/ysia by decreasing a specific potassium current (Klein et al., 1982; californica undergo heterosynaptic facilitation of transmitter Siegelbaum et al., 1982). This potassium current, called the S current, release during sensitization. As in the siphon sensory neu- is distinct from the fast potassium current (Connor and Stevens, rons, the transmitter serotonin produces facilitation and also 1971; Neher, 1971) the calcium-activated potassium current (Meech elicits a slow, decreased conductance excitatory postsyn- and Standen, 1975) and the delayed potassium current (Hodgkin aptic potential (EPSP) in these neurons. Using voltage clamp and Huxley, 1952; Thompson, 1977). The current is non-inactivating, and biochemical analyses, we have found that the slow EPSP and it participates in the resting and action potentials of the sensory in the pleural sensory neurons is due to a decrease in a neurons. -

BI 360: Neurobiology Fall 2015
BI 360: Neurobiology Fall 2015 Problem Sets: Questions and Answers These problems are provided to aid in your understanding of basic neurobiological concepts and to guide your focus for in-depth study. These practice questions are optional and answers will not be graded, although they will be discussed during discussion sections. The topics in these problem sets parallel those covered in lecture. Neurobiological concepts are complex and difficult, and oftentimes are not easily digested at first reading. We suggest that you review the course material several times by reading different texts/papers and talking through your difficulties with us and your fellow classmates. Stick with it B this material can be fully comprehended through persistent and focused effort. Problems listed below are in order of topics covered in lecture. Problem set numbers do not always correspond to specific lectures or weeks of the term. There are 5 problem sets in this series Problem Set 1 (IONS, MEMBRANES & EQUILIBRIA): 1.1 The equilibrium potential for sodium (ENa) = +58 mV if the concentration of Na on the outside of the cell is 10 times the concentration of Na on the inside of the cell. What happens to ENa if the extracellular concentration of sodium ([Na]o) is increased by a factor of 10? factor of 100? decreased by a factor of 10? Explain why ENa changes in the above examples in terms of the balance between chemical and electrical forces across the cell=s plasma membrane. Answer: The Nernst equation is the formula used to calculate the “equilibrium” or “reversal” potential for a particular ion given the relative internal and external concentrations of the ion. -

Negative Shift in the Glycine Reversal Potential Mediated by Aca2ϩ- and Ph-Dependent Mechanism in Interneurons
The Journal of Neuroscience, September 16, 2009 • 29(37):11495–11510 • 11495 Cellular/Molecular Negative Shift in the Glycine Reversal Potential Mediated by aCa2ϩ- and pH-Dependent Mechanism in Interneurons Yuil Kim1 and Laurence O. Trussell2 1Neuroscience Graduate Program, Oregon Health & Science University, and 2Oregon Hearing Research Center/Vollum Institute, Portland, Oregon 97239 Cartwheel cells are glycinergic auditory interneurons which fire Na ϩ- and Ca 2ϩ-dependent spike bursts, termed complex spikes, and which synapse on both principal cells and one another. The reversal potential for glycine (Egly ) can be hyperpolarizing or depolarizing in cartwheel cells, and many cells are even excited by glycine. We explored the role of spike activity in determining Egly in mouse cartwheel cells using gramicidin perforated-patch recording. Egly was found to shift toward more negative potentials after a period of complex spiking or Ca 2ϩ spiking induced by depolarization, thus enhancing glycine’s inhibitory effect for ϳ30 s following cessation of spiking. 2ϩ Combined perforated patch electrophysiology and imaging studies showed that the negative Egly shift was triggered by a Ca -dependent Ϫ Ϫ intracellular acidification. The effect on Egly was likely caused by bicarbonate-Cl exchanger-mediated reduction in intracellular Cl ,as Ϫ Ϫ H2DIDS and removal of HCO3 /CO2 inhibited the negative Egly shift. The outward Cl flux underlying the negative shift in Egly opposed a positive shift triggered by passive Cl Ϫ redistribution during the depolarization. Thus, a Ca 2ϩ-dependent mechanism serves to maintain or enhance the strength of inhibition in the face of increased excitatory activity. (Farrant and Kaila, 2007). -

The Properties and Regulation of Functional Acetylcholine Receptors on Chick Ciliary Ganglion Neurons
The Journal of Neuroscience, November 1967, 7(11): 3612-3622 The Properties and Regulation of Functional Acetylcholine Receptors on Chick Ciliary Ganglion Neurons Joseph F. Margiotta, Darwin K. Berg, and Vincent E. Dionne Departments of Pharmacology and Biology, University of California, San Diego, La Jolla, California 92093 The properties of acetylcholine receptor (AChR) channels are also found in many areas of the nervous system, where they on chick ciliary ganglion neurons in culture were examined mediate synaptic transmission between neurons. Although ini- using patch-clamp recording techniques. Acetylcholine (ACh) tial studies indicated that neuronal AChRs share some of the was applied by rapid microperfusion. Whole-cell current noise basic functional properties of their counterparts on skeletal mus- analysis revealed a single class of functional receptors on cle fibers (Rang, 198 1; Derkach et al., 1983; Ogden et al., 1984) the neurons. Dose-response studies indicated a & of about significant immunological, pharmacological, and structural dif- 36 PM and a Hill coefficient of 1.5-1.7, predicting 2 ACh ferences have also been reported (Patrick and Stallcup, 1977a, binding sites per receptor. Both fast and slow components b; Carbonetto et al., 1978; Ascher et al., 1979; Boulter et al., of receptor desensitization were observed. Single-channel 1986). Because of these differences, rules that govern the reg- recordings from excised outside-out patches of soma mem- ulation of AChR function, number, and distribution in muscle brane exposed to 2-5 PM ACh indicated a single-channel may not apply to neurons. Information about neuronal AChR conductance of 40 pS, a reversal potential of -9 mV, a mean regulation has been limited both because newly developed re- open duration of 1 msec, and an opening probability of 0.34. -

Synapses Models of the Synapse
Department of Mathematical Sciences B12412: Computational Neuroscience and Neuroinformatics Synapses Models of the synapse The contacts of the axon to target neurons are either located on the dendritic tree or directly on the soma, and are known as synapses. Most synapses are chemical contacts, that is, the arrival of an action potential at the synapse induces the secretion of a neurotransmitter that in turn leads to a change in the potential of the membrane of the target neuron. Depending on the type of synapse, an incoming pulse either causes an increase in electrical potential (excitatory synapse) or a decrease (inhibitory synapse). Left: Schematic diagram showing release of neurotransmitter as vesicle membrane fuses with end bulb membrane. Right: Electron micrograph showing how synaptic vesicles fuse with cell membrane as neurotransmiter is discharged. At a synapse presynaptic firing results in the release of neurotransmitters that causes a change in the membrane conductance of the postsynaptic neuron. This postsynaptic current may be written mathematically as Is = gss(Vs - V) where V is the voltage of the postsynaptic neuron, Vs is the membrane reversal potential and gs is a constant. The term Vs - V is often called a shunt as it acts to push the voltage toward the synaptic reversal potential Vs. The variable s corresponds to the probability that a synaptic receptor channel is in an open conducting state. This probability depends on the presence and concentration of neurotransmitter released by the presynaptic neuron. The sign of Vs relative to the resting potential (assumed to be zero) determines whether the synapse is excitatory (Vs > 0) or inhibitory (Vs < 0). -

Neuroscience 201A Exam “Key”, October 7, 2014
Name: _____KEY_________________________ Neuroscience 201A Exam “Key”, October 7, 2014 Question #1 7.5 pts Consider a spherical neuron with a diameter of 20 µm and a resting potential of -70 mV. If the net negativity on the inside of the cell (all of the “excess” anions) were uniformly distributed within the volume of the cell rather than being “glommed” onto the inner surface of the plasma membrane, what would the concentration of these ions be (in M)? Assume that these are all univalent ions. Assume that the capacitance of the plasma membrane is the standard 1 µF/cm2. Page 1 Name: _____KEY_________________________ Question #2 12.5 pts Imagine that you have a squid neuron with a single population of leak channels, which are cation channels with permeability to only Na+ and K+. The concentrations of permeant ions on each side of the membrane are as follows: Ion Na+ K+ Outside 550 mM 20 mM Inside 55 mM 440 mM a) If the resting membrane potential of the cell is -63 mV, calculate gNa/gK for the leak channels. (You are encouraged to use conductances for this problem (not permeabilities). b) If the squid neuron is spherical with a diameter of 200 µm, if the leak conductance of its membrane is 0.3 mS/cm2, and if the conductance of a single leak channel is 10 pS, calculate the average density of leak channels in the membrane, as # per µm2. Page 2 Name: _____KEY_________________________ Question #3 12.5 pts The above figure illustrates a set of current responses (a, lower traces) to voltage steps (a, top traces) for a population of channels in a neuron with physiological solutions bathing the inside and outside of the cell. -
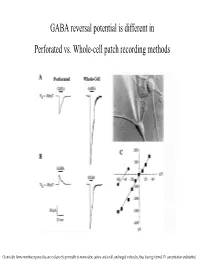
GABA Reversal Potential Is Different in Perforated Vs. Whole-Cell Patch Recording Methods
GABA reversal potential is different in Perforated vs. Whole-cell patch recording methods Gramicidin forms membrane pores that are exclusively permeable to monovalent cations and small, uncharged molecules, thus leaving internal Cl- concentration undisturbed (E GABAA Equilibrium Potential GABAA) is determined by two methods at various ages. Responses shift from monophasic to a more complicated trace due to maturation of GABAB receptor subtype GABAA equilibrium potential declines over development, perhaps due to a fall in chloride ion concentration inside the cell. The chloride ion is the principle ion mediating GABAA receptor effects: the chloride gradient contributes significantly to E GABAA. Whole-cell recordings used to Perforated patch recordings No developmental shift in EGABA bias the internal chloride conc. A Furosemide (chloride to that of the pipette, and muscimol using whole-cell recording. transporter blocker) reduces (agonist) induced reversal potentials GABA equilibrium potential. were measured. These values are A compared to the reversal potential for chloride predicted by Nernst eq. More evidence for GABAA mediated membrane depolarization in early development : GABAA agonist causes reversible calcium entry through voltage-gated Ca++ channels. The reversal potential for endogenously active GABAA-mediated synaptic current (sPSC) is nearly identical to the reversal potential for the exogenously applied GABAA agonist Muscimol. These are not mini’s: TTX abolishes them These sPSC’s are not glutamatergic CNQX and APV do not block. BMI= Bicuculline methiodide, GABAA receptor antagonist. A. whole-cell current clamp B. Perforated patch voltage clamp. Averaged sPSC’s Muscimol induced Spontaneous increases in intracellular calcium (measured by confocal calcium imaging) are mediated by GABAA receptor activation. -

Electrophysiological Properties of Newborn and Adult Rat Spinal Cord
Proc. Nati. Acad. Sci. USA Vol. 91, pp. 3097-3101, April 1994 Neurobiology Electrophysiological properties of newborn and adult rat spinal cord glycine receptors expressed in Xenopus oocytes ANDRAS MORALES*, QUOC-THANG NGUYEN, AND RICARDO MILEDI Laboratory of Cellular and Molecular Neurobiology, Department of Psychobiology, University of California, Irvine, CA 92717 Contributed by Ricardo Miledi, December 30, 1993 ABSTRACT The properties of glycine receptors (GlyRs) quickly frozen in liquid nitrogen and stored at -800C. New- from newborn and adult rat spinal cord were studied in born rats (postnatal days 0-3) were deeply anaesthetized with Xenopus oocytes injected with whole mRNA or the heavy (H) ether and decapitated and spinal cords were dissected as or light (L) mRNA fractions encig their respective GlyRs. above. Mean open times and conductances of channels gated by H- or mINA Isolation and Size Fractionation. mRNA preparation L-GlyRs were determined by noise analysis or voltage jumps. and size separation on sucrose gradients were as described We found that adult H- and L-GlyRs opened channels that (2). Briefly, tissues were homogenized in phenol-saturated differed in their mean open time but had the same channel homogenization buffer (200 mM Tris-HCl/50 mM NaCl/10 conductance. Both H- and L-GlyRs gated Cl currents that mM EDTA/0.5% SDS, pH 9) supplemented with heparin (1 displayed a mir strong outward rectification. Neverthe- mg/ml). The mixture was shaken for 5 min and centrifuged less, single chanls ofadult H-and L-GlyRs did not rectify and for 10 min at 15,300 x g.