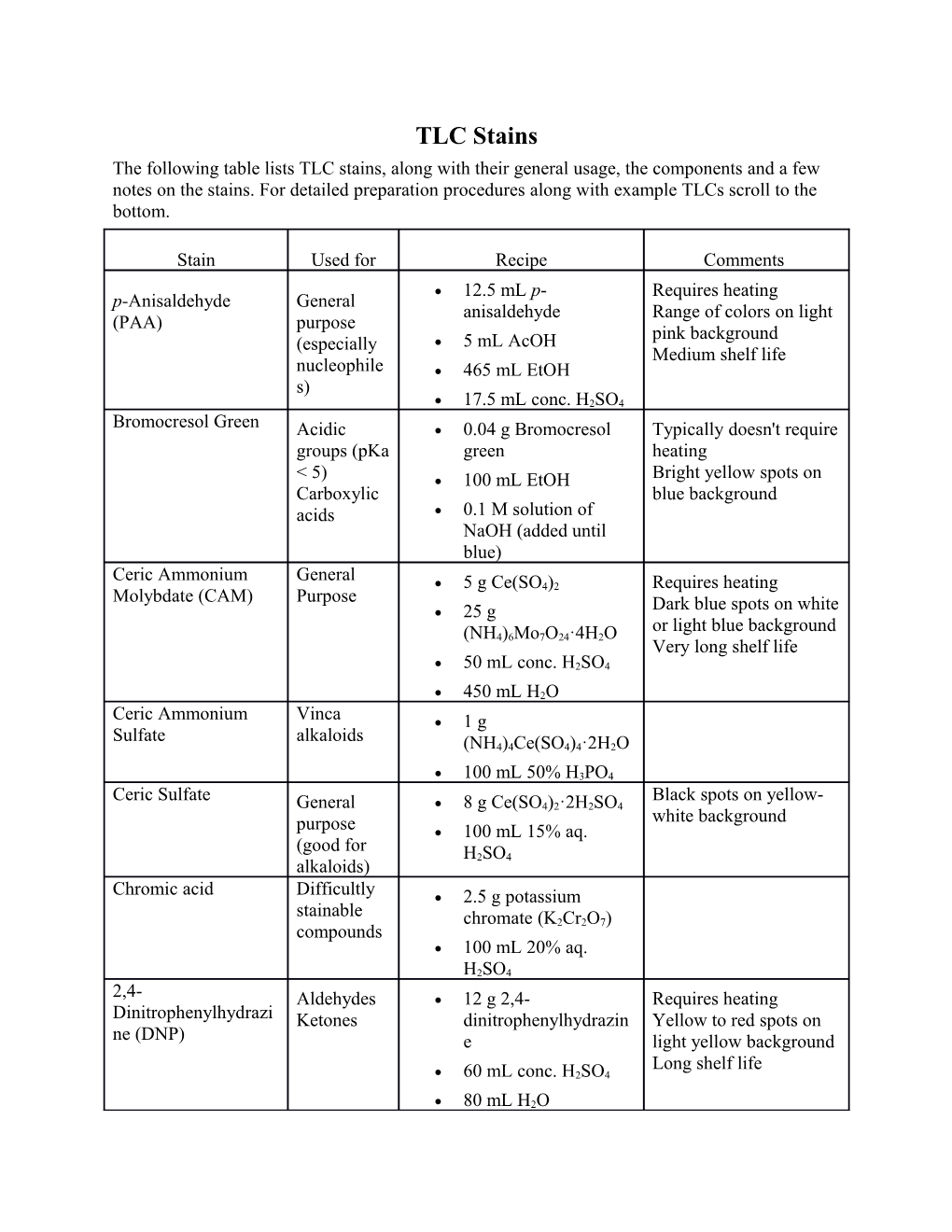
TLC Stains The following table lists TLC stains, along with their general usage, the components and a few notes on the stains. For detailed preparation procedures along with example TLCs scroll to the bottom.
Stain Used for Recipe Comments 12.5 mL p- Requires heating p-Anisaldehyde General anisaldehyde Range of colors on light (PAA) purpose pink background (especially 5 mL AcOH Medium shelf life nucleophile 465 mL EtOH s) 17.5 mL conc. H2SO4 Bromocresol Green Acidic 0.04 g Bromocresol Typically doesn't require groups (pKa green heating < 5) 100 mL EtOH Bright yellow spots on Carboxylic blue background acids 0.1 M solution of NaOH (added until blue) Ceric Ammonium General 5 g Ce(SO4)2 Requires heating Molybdate (CAM) Purpose 25 g Dark blue spots on white or light blue background (NH4)6Mo7O24·4H2O Very long shelf life 50 mL conc. H2SO4
450 mL H2O Ceric Ammonium Vinca 1 g Sulfate alkaloids (NH4)4Ce(SO4)4·2H2O
100 mL 50% H3PO4 Ceric Sulfate Black spots on yellow- General 8 g Ce(SO4)2·2H2SO4 white background purpose 100 mL 15% aq. (good for H2SO4 alkaloids) Chromic acid Difficultly 2.5 g potassium stainable chromate (K2Cr2O7) compounds 100 mL 20% aq. H2SO4 2,4- Aldehydes 12 g 2,4- Requires heating Dinitrophenylhydrazi Ketones dinitrophenylhydrazin Yellow to red spots on ne (DNP) e light yellow background Long shelf life 60 mL conc. H2SO4
80 mL H2O 200 mL 95% EtOH Dragendorff Amines A: Organic 1.7 g base Bi5O(OH)9(NO3)4
80 mL H2O 20 mL AcOH B: 40 g KI
100 mL H2O 5 mL A + 5 mL B in 20 mL AcOH + 70 mL H2O Dragendorff-Munier Amines 10 g KI
1.5 g Bi(NO3)3 20 g Tartaric acid
120 mL H2O Ehrlich Reagent Amines 0.5 g dimethylaminobenzald ehyde
10 mL conc. H2SO4 90 mL 95% EtOH Ferric chloride Phenols 1 g FeCl3 50 mL MeOH
50 mL H2O Iodine Unsaturated A few Iodine crystals Doesn't require heating and Silica Gel Orange to brown spots aromatic on light orange compounds background Spots fade away very rapidly Very long shelf life Morin hydrate General 0.1 g C15H10O7·xH2O Purpose 100 mL MeOH Ninhydrin Amino 0.3 g Ninhydrin acids 3 mL conc. H2SO4 Amines 100 mL n-butanol Phosphomolybdic General 7 g phosphomolybdic Requires heating acid (PMA) purpose acid Dark green/black spots 100 mL EtOH on light green background Long shelf life Potassium Olefins 3 g KMnO4 Alkenes/alkynes/aromati Permanganate Readily cs usually stain without 20 g K2CO3 (KMnO4) oxidized heating groups 5 mL 5% aq NaOH Other oxidizable groups
300 mL H2O require heating Yellow spots on purple background Very long shelf life Vanillin General 15 g vanillin Requires heating purpose 250 mL EtOH Range of colors Medium shelf life 2.5 mL conc. H2SO4 Procedures and examples p-Anisaldehyde (PAA) To a cold (0 °C) mixture of AcOH (5 mL) and absolute EtOH (465 mL) was added p- anisaldehyde (12.5 mL) followed by slow addition of sulfuric acid (17.5 mL). The resulting clear solution was warmed to rt and used as is. The excess was stored in the fridge. This stain is light and oxidation sensitive and will gradually turn pink/orange. The stain should be kept in an aluminum-covered jar while in use, and the excess should be kept cold and in the dark. Once the stain turns dark red, it should be discarded and made fresh again. Ceric Ammonium Molybdate (CAM) - Hanessian's Stain In a dark hood, ceric sulfate (5 g) was added to a cold (0 °C) clear colorless stirring solution of ammonium molybdate (25 g) in water (450 mL). The resulting bright yellow cloudy solution was vigorously stirred and sulfuric acid (50 mL) was slowly added over 90 min resulting in a very exothermic reaction. The resulting clear gold solution was warmed to rt, poured in a jar for immediate use and the excess was stored in the fridge. This stain can be kept for months on the bench and in the fridge. Decoloration to very pale yellow usually occurs but does not affect the stain.
Chromic acid (K2Cr2O7) To a cold (0 °C) solution of sulfuric acid (100 mL, 20% v/v aq.) was slowly added potassium chromate (2.5 g). The resulting clear bright red/orange solution was warmed to rt and used as is. The excess was stored in the fridge. 2,4-Dinitrophenylhydrazine (DNP) To a cold (0 °C) mixture of water (80 mL) and absolute EtOH (200 mL) was added 2,4- dinitrophenylhydrazine (12 g). To the resulting cloudy bright orange solution was slowly added sulfuric acid (60 mL) resulting in a clear orange solution that was warmed to rt and used as is. The excess was stored in the fridge.
Iodine (I2) In a mortar, grind a few crystals of iodine with some silica gel. Transfer to a jar with a plastic screw cap. Spots are visualized by leaving the plate in the chamber for a couple minutes until spots turn brown. Once taken out of the chamber, spots will rapidly fade away. This stain can be kept for months on the bench. Once it fades to white (by I2 sublimation) it should be made fresh again. Phosphomolybdic acid (PMA) Phosphomolybdic acid (10 g) was added to cold (0 °C) absolute EtOH (100 g) and then warmed to rt. The resulting cloudy yellow suspension was filtered over filter paper to give a clear bright yellow solution that was used as is. The excess was stored in the fridge. This stain can be kept for months on the bench and in the fridge. PMA stain will gradually turn green but does not affect the stain.
Potassium Permanganate (KMnO4) In a 1000 mL erlenmeyer flask was added sequentially water (600 mL), 5% aq. NaOH (10 mL) and K2CO3 (40 g). Once the solution became clear, KMnO4 (6 g) was added resulting in a dark purple solution. The solution was stirred 1 h at rt. The resulting purple solution was poured in a jar for immediate use and the excess was stored in the fridge. This stain can be kept for months on the bench and in the fridge. Vanillin To a cold (0 °C) clear colorless solution of vanillin (15 g) in absolute EtOH (250 mL) was slowly added sulfuric acid (2.5 mL). The resulting clear solution was warmed to rt and used as is. The excess was stored in the fridge. This stain is light and oxidation sensitive and will gradually turn dark. The stain should be kept in an aluminum-covered jar while in use, and the excess should be kept cold and in the dark. Once the stain turns black, it should be discarded and made fresh again. Example: TLC plates stained with CAM, PMA, DNP, Vanillin, KMnO4, p-anisaldehyde and I2