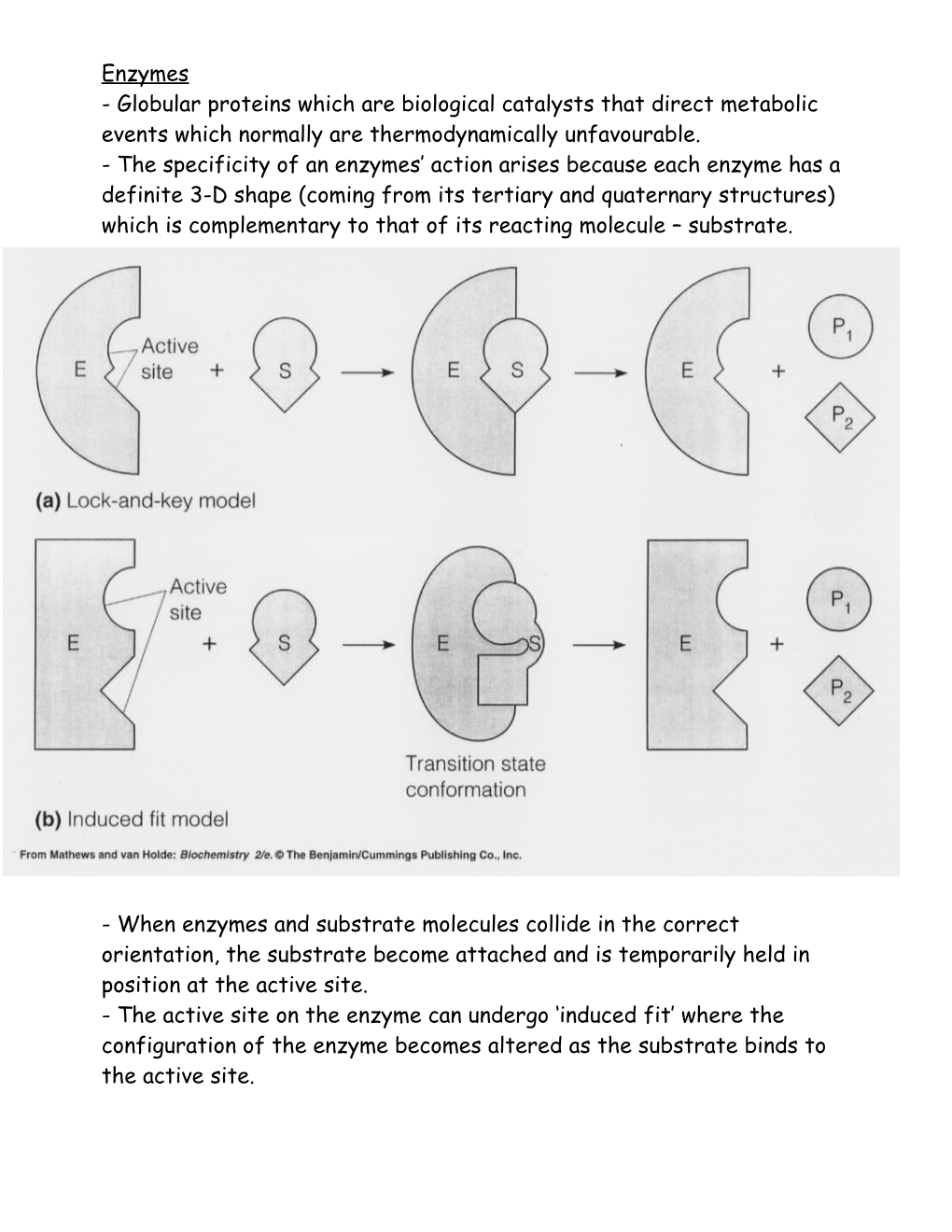
Enzymes - Globular proteins which are biological catalysts that direct metabolic events which normally are thermodynamically unfavourable. - The specificity of an enzymes’ action arises because each enzyme has a definite 3-D shape (coming from its tertiary and quaternary structures) which is complementary to that of its reacting molecule – substrate.
- When enzymes and substrate molecules collide in the correct orientation, the substrate become attached and is temporarily held in position at the active site. - The active site on the enzyme can undergo ‘induced fit’ where the configuration of the enzyme becomes altered as the substrate binds to the active site. Enzyme cofactors - Many enzymes will not work correctly unless a smaller non-protein substance, called a co-factor (Mg2+, Fe2+, Zn2+, Cu2+) is present within the active site. - Alternately the cofactor maybe a small organic molecule. These are called co-enzymes and are closely related to Vitamins.
Enzyme Inhibitors - Inhibition occurs when the action of an enzyme is slowed down by another substance.
1) Competitive Inhibitors – these substrates are chemically related to the substrate and have a shape which is very similar. They compete with the substrate for the active site. - They have no lasting effects on the enzyme; their intervention can be overcome by increasing the concentration of the substrate or reduce the concentration of the inhibitor.
2) Non-competitive Inhibitors – they do not combine with the active site of the enzyme and are unaffected by the substrate concentration. The commonest types include heavy metals (Ag+ and Hg2+). They combine with the enzyme molecules, producing a change which indirectly affects the shape of the active site, so that the substrate no longer fits. Factors Affecting Enzyme Controlled Reactions
1) Temperature – there is an optimum temperature which corresponds to the maximum reaction rate. Above the optimum temperature, the reaction rate falls off sharply. This occurs when the hydrogen bonds in the proteins of the enzymes become unstable, causing denaturation of the enzyme. As a result of this denaturation, the enzyme changes shape and no longer works.
2. pH – optimum pH is close to 7, which is normal intercellular pH. At extreme pH values, the enzymes become denatured.
3. Concentration of substrate and enzyme
- An increase in concentration of substrate will only increase the reaction rate so much, until a point is reached where the reaction rate depends only on the amount of enzyme present. - At low [S], the rate is first order with respect to [S] - nearly a straight line. When substrate is saturated, it becomes zeroeth order (rate not dependent on [S]), as active sites run out.
- In fact, it becomes pseudo-first order with respect to enzyme concentration if the substrate is present in massive excess.
- This leads to the Michaelis-Menten equation, explaining the theory of enzyme kinetics.
k1 k 3
E + S ⇌ ES ⇌ E + P
k2 k 4
- k1 and k3 are forwards rate constants; k2 and k4 are backwards rate constants for the formation of the enzyme-substrate (ES) complex, and for the formation of product (P) respectively.
rate = V = Vmax [S] Vmax – when enzyme is saturated, Km + [S] maximum rate.
Km = (k2 + k3)/ (k1 + k4) - Km is equal to the [S] when the rate is equal to half of the maximum value. (See first figure). - The value of Km is experimentally determined and is dependent on [S], temperature and pH.
- rearranging gives us:
1 = Km x 1 x 1 V Vmax [S] Vmax
Y = m x + b
- This allows the calculation of the maximum rate (Vmax) and Km.