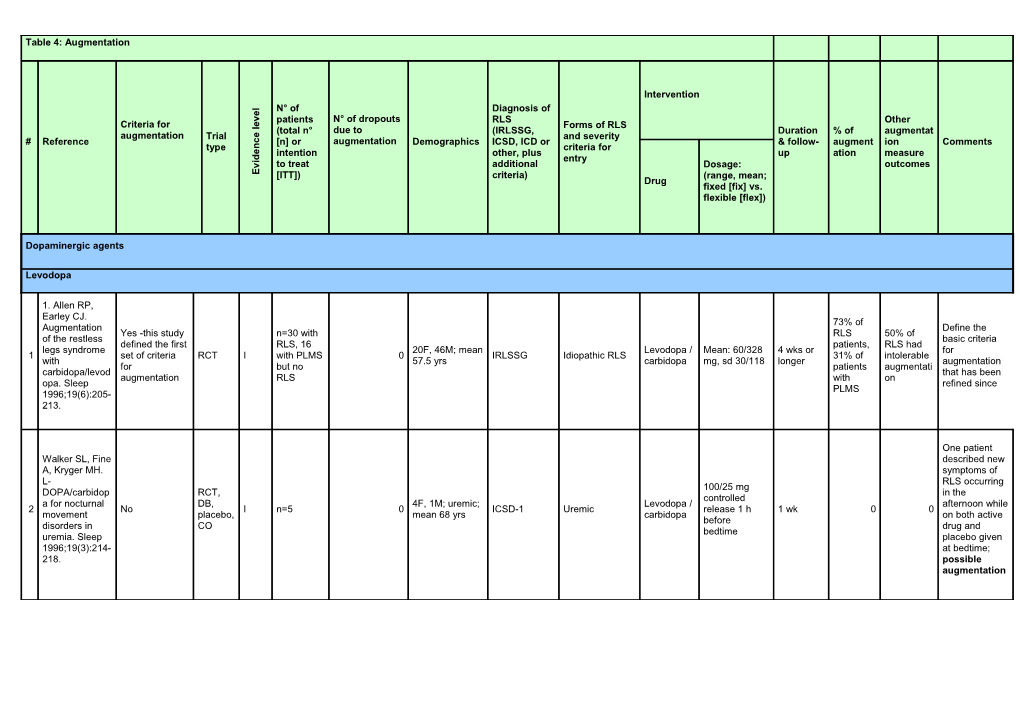
Table 4: Augmentation
Intervention
l N° of Diagnosis of e
v patients N° of dropouts RLS Other e
Criteria for l Forms of RLS (total n° due to (IRLSSG, Duration % of augmentat augmentation Trial e and severity # Reference c [n] or augmentation Demographics ICSD, ICD or & follow- augment ion Comments type n criteria for e intention other, plus up ation measure d
i entry
v to treat additional Dosage: outcomes E [ITT]) criteria) (range, mean; Drug fixed [fix] vs. flexible [flex])
Dopaminergic agents
Levodopa
1. Allen RP, Earley CJ. 73% of Augmentation Define the Yes -this study n=30 with RLS 50% of of the restless basic criteria defined the first RLS, 16 patients, RLS had legs syndrome 20F, 46M; mean Levodopa / Mean: 60/328 4 wks or for 1 set of criteria RCT I with PLMS 0 IRLSSG Idiopathic RLS 31% of intolerable with 57.5 yrs carbidopa mg, sd 30/118 longer augmentation for but no patients augmentati carbidopa/levod that has been augmentation RLS with on opa. Sleep refined since PLMS 1996;19(6):205- 213.
One patient Walker SL, Fine described new A, Kryger MH. symptoms of L- RLS occurring 100/25 mg DOPA/carbidop RCT, in the controlled a for nocturnal DB, 4F, 1M; uremic; Levodopa / afternoon while 2 No I n=5 0 ICSD-1 Uremic release 1 h 1 wk 0 0 movement placebo, mean 68 yrs carbidopa on both active before disorders in CO drug and bedtime uremia. Sleep placebo given 1996;19(3):214- at bedtime; 218. possible augmentation 26.7% reported a time shift of RLS symptom s in the afternoon or evening The major during characteristic the was an altered treatment pattern of RLS with rr- symptoms. The Collado-Seidel levodopa presence of all V, Kazenwadel and sr- 4 J, Wetter TC, et rr-Levodopa levodopa. augmentation al. A controlled (100 or 200 16.7% criteria was not study of mg) for 2 wks reported reported. A additional sr-L- followed by a time time shift of IRSSG + dopa in L-dopa- Levodopa/b additional sr- shift RLS symptoms RCT, 19F, 11M; mean PLMSI > 5 + Idiopathic & 3 responsive No I n=30 0 enserazide, Levodopa/ben 4 wks during No seems to be a DB, CO 58 yrs SL > 25 min + secondary RLS restless legs placebo serazide the predictive SE ≤ 85% syndrome with 100/25 mg or therapy factor for an late-night 200/50 mg or with rr-L- increase in symptoms. placebo, 1hr dopa and severity of RLS Neurology before bedtime placebo. symptoms. 1999;52(2):285- 1 patient 75% of 290. experienc patients with a ed the time shift of time shift symptoms had in both taken levodopa treatment during the year periods. 2 before the trial patients reported other limbs were involved in RLS symptom s Staedt J, Wassmuth F, Ziemann U, Hajak G, Ruther E, Stoppe G. Pergolide: treatment of choice in restless legs Rebound syndrome Pergolide phenomenon ( RLS) and 0.125 mg at 18 days in the second Pergolide, nocturnal RCT, 5F, 6M; 50-69 bedtime vs. for each half of the night 4 No 1 n=11 0 Not specified Idiopathic RLS levodopa / Unknown No myoclonus DB, CO yrs levodopa / active mentioned for carbidopa syndrome carbidopa 250 drug levodopa; no (NMS). A mg mention of double -blind augmentation randomized crossover trial of pergolide versus L-Dopa. J Neural Transm 1997;104(4- 5):461-468.
Data do not support the relevance of augmentation in the Benes H, treatment of Kurella B, RLS by Kummer J, levodopa / Kazenwadel J, benserazide. Selzer R, The short Kohnen R. treatment Rapid onset of ISGRLS + Levodopa / period of 4 wks action of RCT, 19F, 13M; SL>30m Levodopa / benserazide and the low levodopa in DB, Idiopathic and 5 No I n=32 0 mean age 56 and/or SE≤ benserazid 100/25 mg 1 or 4 wks 0 No dose of restless legs placebo, uremic RLS yrs 85% and e 2 cps 1hr levodopa / syndrome: a CO, MC PLMAI > 5/hr. before bedtime benserazide double-blind, (max. 200 / 50 randomized, mg) might also multicenter, explain why we crossover trial. did not observe Sleep time-shift 1999;22(8):107 problems in 3-1081. our trial. Augmentation not specifically looked for Eisensehr I, Ehrenberg BL, Rogge Solti S, Noachtar S. Treatment of idiopathic RCT, IRLSSG 20% restless legs DB drug sr valproate 12F, 8M, mean PLMS 10/h (4/20 syndrome & 600 mg; sr Augmentation age 58.9 yrs TST and RLS Valproate, 3 wks augment 6 (RLS) with No placebo, I n=20 0 Idiopathic levodopa / No not specifically (6.9), range 41- daily for at levodopa each ation on slow-release CO, benserazide looked for 74 yrs least 6 levodopa valproic acid followed 200/50 mg months. sr) compared with by open slow-release levodopa/bense razid. J Neurol 2004;251:579- 583.
Cabergoline 2 Trenkwalder C, mg vs. Benes H, Grote levodopa 200 L, et al. mg for 6 wks; Cabergoline If not compared to 14.2 % efficacious, levodopa in the with cabergoline treatment of augment RCT, Cabergoline: Cabergolin increased up patients with Yes, 9.8% in ation in Earley and DB, 67% F; mean IRLSSG with e, to 3 mg and Specifically severe restless levodopa vs. levodopa; 7 Allen criteria + prosp, I n=361 56,9 yrs. score IRLS at Idiopathic levodopa / levodopa up to 30 wks asked about legs syndrome: 3.9% in vs. 5.6 % the ASRS parallel, Levodopa: 75% least 10 benserazid 300 mg. augmentation Results from a cabergoline. on MC F; 58.7 yrs e Cabergoline multi-center, cabergoli given 3 hrs randomized, ne, P = before active 0.0104 bedtime, controlled trial. levodopa in 2 Mov Disord doses, 3 hrs 2007;22(5):696- before and at 703. bedtime
Ergot-derived dopamine agonists
Pergolide Earley CJ, Allen RP. Pergolide 17.4% and (i.e. 4/23 Allen RP, carbidopa/levod not 15% Earley CJ. opa treatment as Augmentation of the restless Not adequately mentione of the restless flexible, 1-2 legs syndrome treated with d in the Clinical legs syndrome Open, doses per day, 18 (2-39) 1 and periodic leg III n=23 1 not specified IRLSSG levodopa / Pergolide paper assessment of with prosp. maximum 1 months movements in carbidopa since the investigator carbidopa/levod mg sleep in a (400/100 mg) number opa. Sleep consecutive included 1996;19(3):205 series of the PLMS -213. patients. Sleep only 1996;19(10):80 patients) 1-810.
Development of Silber MH, RLS symptoms Shepard JW, in the evening Jr., Wisbey JA. or afternoon or flexible, 0.05 - Pergolide in the an increase of "Problematic 0.75 in the See criteria management of 15F15, 5M; 41- severity of pre- OL, RLS" (n=13 evening, 2nd 25 (6- 28) for Heterogeneous 2 restless legs III n=20 0 86 yrs; mean 63 IRLSSG Pergolide 27% existing prosp. augmentation and 3rd dose months augmentati patient group syndrome: an yrs symptoms at under levodopa) during the day on extended study. those times possible Sleep compared to 1997;20(10):87 before therapy 8-882. with the drug
Stiasny K, Wetter TC, Allen RP, Winkelmann J, Earley CJ. et al. Long-term Augmentation effects of of the restless Idiopathic RLS, 426.4 ± Clinical pergolide in the legs syndrome OL, n=22; idiopathic RLS; completers of Flexible, 0.37 ± 92.3 days 3 III 0 IRLSSG Pergolide 27.3% no assessment of treatment of with prosp. ITT=28 16F, 12M DB trial Wetter 0.15 mg (range: investigator restless legs carbidopa/levod et al. 1999 316 - 665) syndrome. opa. Sleep Neurology 1996;19(3):205 2001;56(10):13 -213. 99-1402.
Cabergoline
1 Trenkwalder et al. 2007 see levodopa
Oertel et al. Efficacy of cabergoline in restless legs syndrome: a RCT, placebo- DB, Idiopathic RLS, Augmentation controlled study prosp., IRLS > 10, RLS- Cabergolin 2 Not specified I n=40 0 Idiopathic RLS IRLSSG Fixed 2 mg 5 wks 0 no not specifically with parallel, 6 at night > 4, e looked for polysomnograp MC, PLMSAI > 5 hy placebo (CATOR).Neuro logy 2006;67:1040- 1046
Stiasny-Kolster K, Benes H, Allen RP, 3% Open, Peglau I, et al. Earley CJ. ( 2/66); prosp, Effective Augmentation including MC of cabergoline of the restless Completion of those an RCT, Careful review treatment in legs syndrome RCT, DB, Cabergolin Flexible up to 7 with 3 DB, III n=66 1 idiopathic RLS IRLSSG 47 wks no of patient idiopathic with prosp., parallel, e mg possible prosp., charts restless legs carbidopa/levod MC, placebo augment parallel, syndrome. opa. Sleep ation MC, Neurology 1996;19(3):205 9.1% (2 placebo 2004;63:2272- -213. +4 of 66) 2279.
Stiasny K, Robbecke J, idiopathic RLS, Schuler P, Allen RP, previously had Oertel WH. Earley CJ. been treated Treatment of Augmentation insufficiently idiopathic of the restless Idiopathic RLS, with levodopa Flexible, mean restless legs Specifically legs syndrome OL, 7F, 2M; 38.1- despite several Cabergolin dosage 2.1 syndrome III n=9 0 IRLSSG 12 wks 0 no asked about with prosp. 64.3 yrs; mean attempts of e mg, range 1-4 (RLS) with the augmentation. carbidopa/levod 54.1 yrs adjustment and mg D2-agonist opa. Sleep in part cabergoline--an 1996;19(3):205 developed open clinical -213. augmentation trial. Sleep. under levodopa 2000 May 1;23(3):349-54. Benes H, Heinrich CR, Ueberall MA, Kohnen R. Long-term Allen RP, safety and Earley CJ. efficacy of Augmentation cabergoline for of the restless 222F; 80M, Clinical the treatment of legs syndrome OL, Idiopathic Cabergolin Flexible up to 3% 4 III n=302 0 mean age 61 no 26 wks no assessment of idiopathic with prosp. RLS e 3.5 mg (9/302) yrs investigator restless legs carbidopa/levod syndrome: opa. Sleep results from an 1996;19(3):205 open-label 6- -213. month clinical trial. Sleep 2004;27:674- 682.
Lisuride
Benes H Pretreat Transdermal Allen RP, 10 IRLS; ment lisuride: short- Earley CJ. 3 RLS-6 severity 2 wks term efficacy Augmentation of RLS during OL, OL flexible and tolerability of the restless the day; 2 wks OL followed 3F, 4M; 18-75 lisuride 3-6 Clinical study in legs syndrome n=9; ITT previous positive run-in, 1 1 by 1 wk I 0 yrs; mean 58± 6 IRLSSG Lisuride mg, fixed dose 0 0 assessment of patients with with =10 wk DB RCT, yrs 3-6 mg in DB investigator severe restless carbidopa/levod levodopa placebo DB, period legs syndrome. opa. Sleep response; prosp., Sleep Med. 1996;19(3):205 response to placebo, 2006 -213. lisuride in OL parallel Jan;7(1):31-5.
Non-ergot derived dopamine agonists
Ropinirole Augmentation Bogan RK, Fry spontaneously JM, Schmidt reported as AE MH, Carson 3 patients. SW, Ritchie SY. Ropinirole Ropinirole in (1.6%) the treatment of 1 patient on patients with 381; MC, Idiopathic RLS placebo restless legs ITT=321 109F, ropinirole: DB, excluded if signs (0.5%) had syndrome: a (187) 164 58,3% F; Ropinirole, Not 1 Not specified placebo, I 0 IRLSSG of secondary 0.25-4.0 mg/d 12 wks 0 reports with the US-based ropinirole placebo: 63.7% placebo defined flexible- forms, term randomized, (193) 167 F; 18-79 yrs dose augmentation augmentation double-blind, placebo noted. These placebo- events controlled occurred clinical trial. during the Mayo Clin Proc treatment 2006;81(1):17- phase of the 27. study
Allen R, Becker PM, Bogan R, et al. Ropinirole decreases Idiopathic RLS "The periodic leg n=65 5 PLMS/hr worsening of movements and DB, ITT=59 IRLS >15 Ropinirole, RLS symptoms 2 improves sleep Not specified parallel, I (with RLS 0 18-79 yrs IRLSSG minimum of 15 0.25-4.0 mg/d 12 wks 0 no placebo has been parameters in placebo and nights with RLS linked to patients with PLMS) symptoms in the augmentation." restless legs month prior syndrome. Sleep 2004;27 907-914. Walters AS, Ondo WG, Dreykluft T, Primary Grunstein R, diagnosis of Patients were Lee D, Sethi K. RLS, only excluded if Ropinirole is idiopathic they suffered effective in the score > 15 on from treatment of IRLS augmentation restless legs DB, There were no 18-79 yrs; at least 15 syndrome. RCT, 0.25-4.0 mg/d n=267 reports of Australia, nights of RLS Ropinirole, Although 3 TREAT RLS 2: Not specified MC, I IRLSSG 1 to 3 hrs 12 wks 0 no ITT=266 augmentation Europe, North symptoms placebo augmentation a 12-week, parallel, before bedtime during treatment America during the was not double-blind, placebo previous month systematically randomized, pat. who would measured or parallel-group, have required directly placebo- daytime assessed in controlled treatment was this trial study. Mov excluded Disord 2004;19(12):14 14-1423.
Trenkwalder C, Garcia- Borreguero D, Montagna P, et al. Ropinirole in the treatment of restless legs Idiopathic, IRLS syndrome: > 15, Patients were results from the had either excluded if TREAT RLS 1 experienced at they were study, a 12 least 15 nights RCT, 18-79 yrs; 10 0.25–4.0 mg/d experiencing week, IRLSSG with symptoms Ropinirole, 4 No information DB, 1 ITT=284 0 European 3 hrs before 12 wks 0 no augmentation randomised, IRLS >15 of RLS in the placebo placebo countries bedtime placebo previous month There were no controlled study or, reported they reports of in 10 European had symptoms augmentation countries. J of this frequency Neurol before treatment Neurosurg Psychiatry 2004;75(1):92- 97. Montplaisir J, Karrasch J, Mean and Haan J, Volc D. median dose Single Ropinirole is n=202 51F; 41M; 18-79 at wk 20, after blind effective in the (single yrs which no more Idiopathic RLS phase: 24 long-term blind 18 centers in IRLSSG changes in 1.5% (3 Study was not RCT, 15 nights of RLS wks management of phase) Australia, Essential Ropinirole, dose were possible specifically 5 No placebo, I 1 symptoms double restless legs ITT=n=92 Austria, Criteria placebo allowed: 2.05 augment designed to MC during the blind syndrome: a (double Canada, IRLS >15 and 2.00 mg/d ation) assess the previous month phase: for randomized blind Germany, and at wk 24: a further controlled trial. phase) South Africa 15.8% of pat. 12 wks Mov Disord max. dose of 2006;21(10):16 4.0 mg/d 27-1635.
Pramipexole
Kaplan-Meier plot indicated that 20% of patients had developed augmentation after 1 yr of treatment and 30% after 2 yrs. Risk of Defined as the augmentation Silber MH, new tapered off Girish M, Develope development or Mean daily after 2.5 yrs. Izurieta R. 21 (25%) d in 33% increase in 1 patient dose when prior Pramipexole in IRLSSG idiopathic RLS Range 4 - after a severity, discontinued augmentati augmentation the (PLMS "reason for 46 months mean of duration, or pramipexole 36F, 24M; 25-82 Mean 0.63 mg on with either management of Retro. Index>10 (28 changing Pramipexol mean 13.8 1. anatomic III n=60 after more than yrs; mean 57.7 range 0.25-4.5 developed carbidopa / restless legs review pat., who medications e duration months distribution of 4 months yrs mg was 0.56 levodopa or syndrome: an underwent were daytime 27.2 treatment RLS earlier in because of mg (range pergolide was extended study. polys.)) augmentation, ... months (range 2- the day than augmentation 0.25-1.0 not associated Sleep " 31 the time a mg) with a 2003;26:819- months) medication for significant 821. RLS was taken increased risk for augmentation with pramipexole. Augmentation was generally treated with additional doses earlier in the day Augmentation was defined as the need for earlier (by at least 2hrs) administration of the same Winkelman JW, dose of Johnston L. pramipexole, Clinical Augmentation due to an Idiopathic RLS Mean time predictors were and tolerance earlier n=18, 0.125- (78%) At least 6 to the first previous with long-term appearance of 0.25 mg; n=29, Retro. 58% F, 42% M; secondary RLS months 32% episode of augmentation pramipexole RLS symptoms n=72 Pramipexol 0.375-0.5 mg; 2. assess III 1 31-91 yrs; mean IRLSSG (22%) mean (19/59) augmentati (P=0.06) or treatment of (at least 5 times ITT=59 e n=9 0.625-0.75 ment 60.8+14.4 yrs fam. RLS (20%) duration: (P>0.05) on was 8.8 previous restless legs per wk), or a mg; n=3, 1.0 Ferritin level 21.2+11.4 months tolerance to syndrome need to mg or more above 40 (+6.5) levodopa (RLS). Sleep increase (P=0.01) Med 2004;5:9- pramipexole 14. dose due to an extension of RLS symptoms beyond the originally affected area (e.g. from legs to arms)
Montplaisir J, Denesle R, Optimal Pramipexole Petit D. OL, Patient who dosage was treatment was Pramipexole in follow- participated in 0.25 mg for 3F, 4M; 43-62 Mean: 7.8 not associated the treatment of up a previous Pramipexol one patient, 4. Not specified III n=7 0 yrs; mean No information months (+ 0 no with morning restless legs (Montpl RCT e 0.5 mg for five 55.4+8.1 yrs 3 months) rebound or syndrome: a aisir, (Montplaisir, patients, 0.75 afternoon follow-up study. 1999) 1999) mg for one augmentation Eur J Neurol patient. 2000;7:27-31. Becker PM, Ondo W, "RLS Sharon D. augmentation Encouraging was not initial response Idiopathic & Pramipexol 1-5 reported in the 5. of restless legs Not specified OL III n=23 0 11F, 12M IRLSSG individualized 0 no secondary RLS e months 19 pat. who syndrome to were treated pramipexole. for 3 months or Neurology longer" 1998;51:1221- 1223.
(1) Patients. using other medications at the time of study initiation (12 clonazepam, 3 levodopa) were not allowed to change their doses (2) no correlation between serum ferritin levels 8.3% Ferini-Strambi and Dose was (one with L. Restless legs Mean age augmentation. variable: idiopathic syndrome 58yrs (49- (3) 0.25 mg in 40 RLS, 4 augmentation Idiopathic RLS 75) Augmentation IRLSSG patients, with and 37–90 yrs; (n=39) Pramipexol mean RLS occurs within 6. Not specified OL III n=60 No information only nighttime 0.5 mg in 6 months secondar pramipexole mean 58 yrs secondary RLS e duration of the first 4 symptoms seven patients, y RLS) treatment. (n=21) 24 yrs months of after 4-15 Sleep Med (range 9- treatment. and 1.0 mg in wks of 2002;3 29) (4) is not 13 patients treatment Suppl:S23-25. influenced by . the severity of RLS before the onset of pramipexole treatment. (5) seems to be unrelated to the dose of medication (6) low optimal therapeutic dose 0.25 mg/d in most RLS patients (66%) Trenkwalder C, Stiasny-Kolster K, Kupsch A, Oertel WH, An onset of Koester J, symptoms Found no Reess J. progressively cases of RCT, (6-months Augmentation Controlled earlier in the aug. DB, Mean 0.50 mg run-in was withdrawal of day, but 18-80 yrs (Augment parallel, Idiopathic RLS individually period) considered an pramipexole sometimes an mean age 59.6 Pramipexol ation 7 placebo, I n=150 0 IRLSSG IRLS total score optimized dose no AE, but in this after 6 months expansion of (SD 10.3) e, placebo Severity MC, at baseline > 15 of 0.125-0.75 (for a population of of open-label symptoms from 72.8%F Rating withdra mg/d further 3 responders it treatment in the legs to the Scale wal month) did not occur. patients with arms or trunk, was restless legs or even the used) syndrome. Mov entire body Disord 2006;21:1404- 1410.
Oertel WH, Stiasny-Kolster K, Bergtholdt B, et al. Pramipexole Starting dose RLS Study Idiopathic RLS of 0.125 mg/d Group. Efficacy 222F, 116M; According to RLS symptoms max. of 0.75 of pramipexole RCT, 18-80 yrs patient report, Increase in present for at mg/d in restless legs DB, pts. from IRLSSG Pramipexol augmentation 8 daytime I n=345 0 least 2 -3 dose was 6 wks 0 no syndrome: a placebo, 37centers in 5 IRLS >15 e, placebo was not symptoms days/wk in the 3 individually six-wk, MC European specifically month before optimized multicenter, countries assessed study entry according to randomized, PGI double-blind study (effect- RLS study). Mov Disord 2007;22:213-9.
Pribedil Effective dose Earlier onset of ranged from RLS symptoms 3/13 (23%) 25-350 mg/d during the idiopathic RLS; mean 120 Evidente VG. evening, 4/13 (31%) PD; mg/d; l; to Piribedil for shorter latency and 6/13 (46%) prevent restless legs to onset after 6F, 7M; 39–87 clinical signs of nausea and syndrome: a 0-15 3. assuming a OL, pilot III n=13 0 yrs; IRLSSG neuropathy Piribedi vomiting, 0 no pilot study. Mov months restful position, mean 66 yrs (sensory deficits 10–20 mg of Disord increased in the lower domperidone 2001;16:579- intensity, or limbs); no was given 30 81. extension of the patient had iron min before symptoms to deficiency every the upper body dose of piribedil
Abbreviations of trial type: Randomized controlled trial (RCT) Nonrandomized controlled trial (Non-RCT) Open label (open) Double-blind (DB) Single-blind (SB) Prospective (prosp) Retrospective (retro) Crossover (CO) Parallel group (parallel) Placebo controlled (placebo) Single center (SC) Multicenter (MC) Case series (CS)