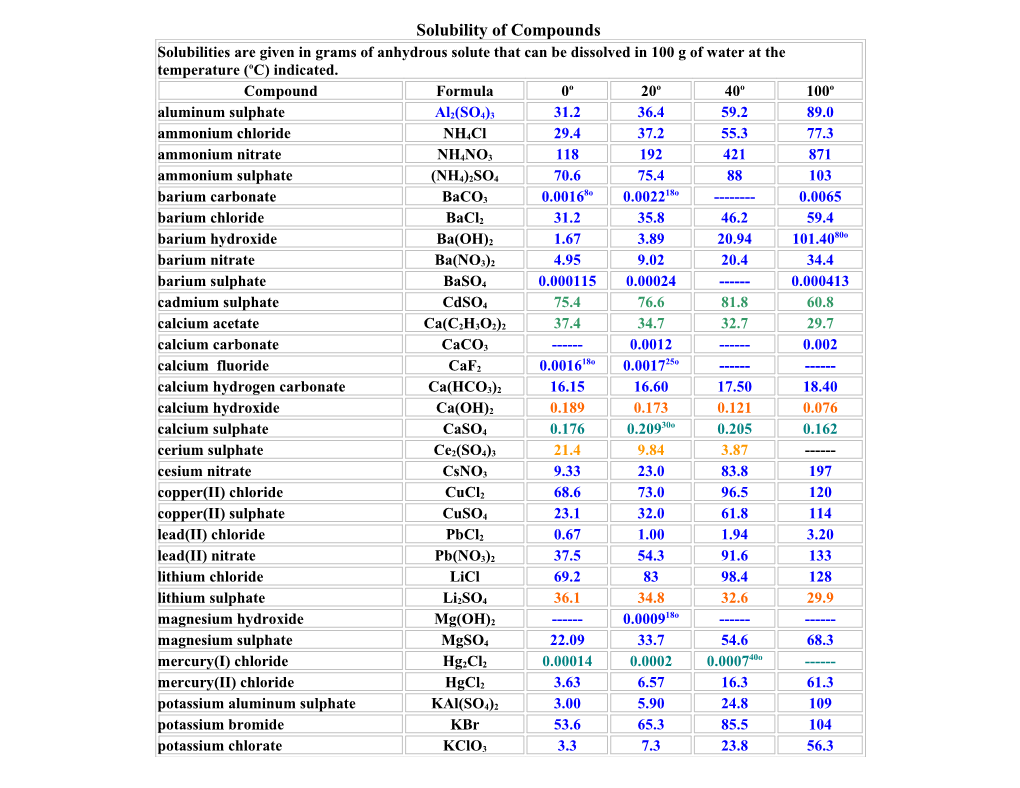
Solubility of Compounds Solubilities are given in grams of anhydrous solute that can be dissolved in 100 g of water at the temperature (oC) indicated. Compound Formula 0o 20o 40o 100o aluminum sulphate Al2(SO4)3 31.2 36.4 59.2 89.0 ammonium chloride NH4Cl 29.4 37.2 55.3 77.3 ammonium nitrate NH4NO3 118 192 421 871 ammonium sulphate (NH4)2SO4 70.6 75.4 88 103 8o 18o barium carbonate BaCO3 0.0016 0.0022 ------0.0065 barium chloride BaCl2 31.2 35.8 46.2 59.4 80o barium hydroxide Ba(OH)2 1.67 3.89 20.94 101.40 barium nitrate Ba(NO3)2 4.95 9.02 20.4 34.4 barium sulphate BaSO4 0.000115 0.00024 ------0.000413 cadmium sulphate CdSO4 75.4 76.6 81.8 60.8 calcium acetate Ca(C2H3O2)2 37.4 34.7 32.7 29.7 calcium carbonate CaCO3 ------0.0012 ------0.002 18o 25o calcium fluoride CaF2 0.0016 0.0017 ------calcium hydrogen carbonate Ca(HCO3)2 16.15 16.60 17.50 18.40 calcium hydroxide Ca(OH)2 0.189 0.173 0.121 0.076 30o calcium sulphate CaSO4 0.176 0.209 0.205 0.162 cerium sulphate Ce2(SO4)3 21.4 9.84 3.87 ------cesium nitrate CsNO3 9.33 23.0 83.8 197 copper(II) chloride CuCl2 68.6 73.0 96.5 120 copper(II) sulphate CuSO4 23.1 32.0 61.8 114 lead(II) chloride PbCl2 0.67 1.00 1.94 3.20 lead(II) nitrate Pb(NO3)2 37.5 54.3 91.6 133 lithium chloride LiCl 69.2 83 98.4 128 lithium sulphate Li2SO4 36.1 34.8 32.6 29.9 18o magnesium hydroxide Mg(OH)2 ------0.0009 ------magnesium sulphate MgSO4 22.09 33.7 54.6 68.3 40o mercury(I) chloride Hg2Cl2 0.00014 0.0002 0.0007 ------mercury(II) chloride HgCl2 3.63 6.57 16.3 61.3 potassium aluminum sulphate KAl(SO4)2 3.00 5.90 24.8 109 potassium bromide KBr 53.6 65.3 85.5 104 potassium chlorate KClO3 3.3 7.3 23.8 56.3 potassium chloride KCl 28.0 34.2 45.8 56.3 potassium chromate K2CrO4 56.3 63.7 70.1 75.6 potassium iodide KI 128 144 176 206 potassium nitrate KNO3 13.9 31.6 106 245 potassium KMnO 2.83 6.34 22.1 ------permanganate 4 potassium sulphate K2SO4 7.4 11.1 18.2 24.1 80o silver acetate AgC2H3O2 0.73 1.05 1.93 2.59 silver chloride AgCl 0.00007 0.000194 0.000550o 0.002 silver nitrate AgNO3 122 216 440 733 sodium acetate NaC2H3O2 36.2 46.4 139 170.15 sodium chlorate NaClO3 79.6 95.9 137 204 sodium chloride NaCl 35.7 35.9 37.1 39.2 sodium nitrate NaNO3 73.0 87.6 122 180 sucrose C12H22O11 179.2 203.9 287.3 487.2 30o ytterbium sulphate Yb2(SO4)3 44.2 22.2 10.4 4.7
Solubility of Gases in Water
Volume of gas (reduced to STP) that can be dissolved in 1 volume of water at the temperature (oC) indicated. Gas 0o 10o 20o 60o air 0.02918 0.02284 0.01868 0.01216 ammonia 1130 870 680 200 carbon dioxide 1.713 1.194 0.878 0.359 carbon monoxide 0.03537 0.02816 0.02319 0.01488 chlorine 4.54 3.148 2.299 1.023 hydrogen 0.02148 0.01955 0.01819 0.01600 hydrogen chloride 512 475 442 339 hydrogen sulphide 4.670 3.399 2.582 1.190 nitrogen 0.02354 0.01861 0.01545 0.01023 nitrogen dioxide 0.07381 0.05709 0.04706 0.02954 oxygen 0.04889 0.03802 0.03102 0.01946 sulphur dioxide 79.789 56.647 39.374 ------