Supplemental information
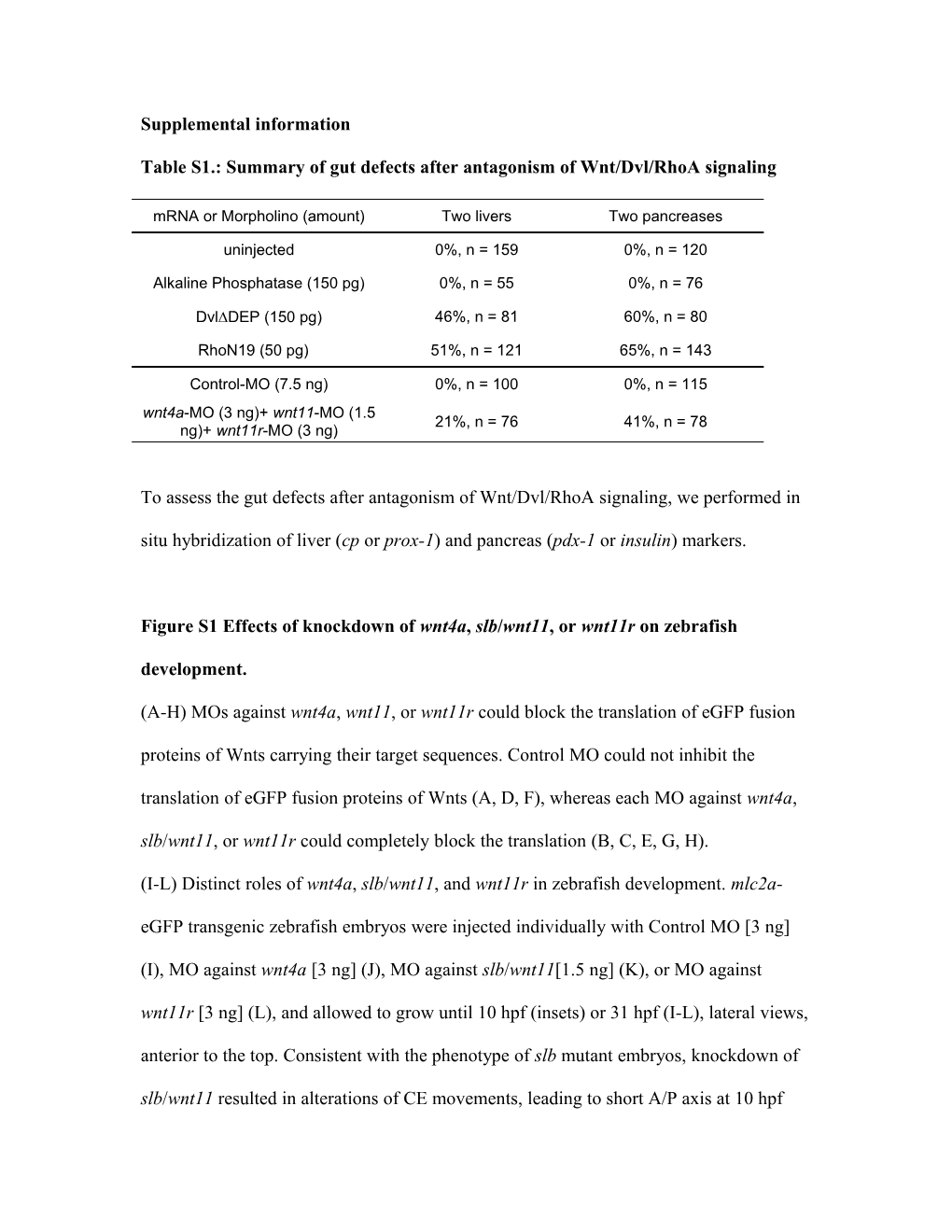
Table S1.: Summary of gut defects after antagonism of Wnt/Dvl/RhoA signaling
mRNA or Morpholino (amount) Two livers Two pancreases
uninjected 0%, n = 159 0%, n = 120
Alkaline Phosphatase (150 pg) 0%, n = 55 0%, n = 76
DvlDEP (150 pg) 46%, n = 81 60%, n = 80
RhoN19 (50 pg) 51%, n = 121 65%, n = 143
Control-MO (7.5 ng) 0%, n = 100 0%, n = 115 wnt4a-MO (3 ng)+ wnt11-MO (1.5 21%, n = 76 41%, n = 78 ng)+ wnt11r-MO (3 ng)
To assess the gut defects after antagonism of Wnt/Dvl/RhoA signaling, we performed in situ hybridization of liver (cp or prox-1) and pancreas (pdx-1 or insulin) markers.
Figure S1 Effects of knockdown of wnt4a, slb/wnt11, or wnt11r on zebrafish development.
(A-H) MOs against wnt4a, wnt11, or wnt11r could block the translation of eGFP fusion proteins of Wnts carrying their target sequences. Control MO could not inhibit the translation of eGFP fusion proteins of Wnts (A, D, F), whereas each MO against wnt4a, slb/wnt11, or wnt11r could completely block the translation (B, C, E, G, H).
(I-L) Distinct roles of wnt4a, slb/wnt11, and wnt11r in zebrafish development. mlc2a- eGFP transgenic zebrafish embryos were injected individually with Control MO [3 ng]
(I), MO against wnt4a [3 ng] (J), MO against slb/wnt11[1.5 ng] (K), or MO against wnt11r [3 ng] (L), and allowed to grow until 10 hpf (insets) or 31 hpf (I-L), lateral views, anterior to the top. Consistent with the phenotype of slb mutant embryos, knockdown of slb/wnt11 resulted in alterations of CE movements, leading to short A/P axis at 10 hpf (inset in K) as well as 31 hpf and partially fused eyes at 31 hpf (K). In contrast, knockdown of wnt4a or wnt11r resulted in defects of CE movements (J, L), milder than those of slb/wnt11-MO-injected embryos. In addition, defects of yolk extension toward the tail were observed in embryos injected with wnt4a-MO (J).
Figure S2 Cardia bifida mutations do not result in a Y-shaped gut tube
(A-F) Expression of mlc2a-eGFP and pdx-1 in 48 hpf wild-type, oep mutant, and mil mutant embryos (A-C, and D-F, respectively). Embryo views are ventral (A-C) and dorsal (D-F), anterior to the top. Both oep and mil mutant embryos display a cardia bifida phenotype (B, C); however, while oep embryos lack anterior endoderm derivatives (red asterisks in E), mil embryos show normal endoderm morphogenesis (F). ib, intestinal bulb. Arrows point to pancreas buds.
Supplementary movie 1: Non-canonical Wnt signaling regulates myocardial migration. Time-lapse imaging of myocardial migration in Control (A), or DvlDEP- injected (B) embryos. The images of Figure 5 A-F represent still frames from these movies.
Supplementary movie 2: Heart beating in embryos injected with DvlDEP. mlc2a-eGFP transgenic zebrafish embryo injected with DvlDEP and allowed to develop until 28 hpf. Injection of DvlDEP results in a clear cardia bifida phenotype, in which both bilateral hearts display distinct atrial and ventricular chambers. Note that, as in other cases of cardia bifida, each heart beats with its own rhythm.