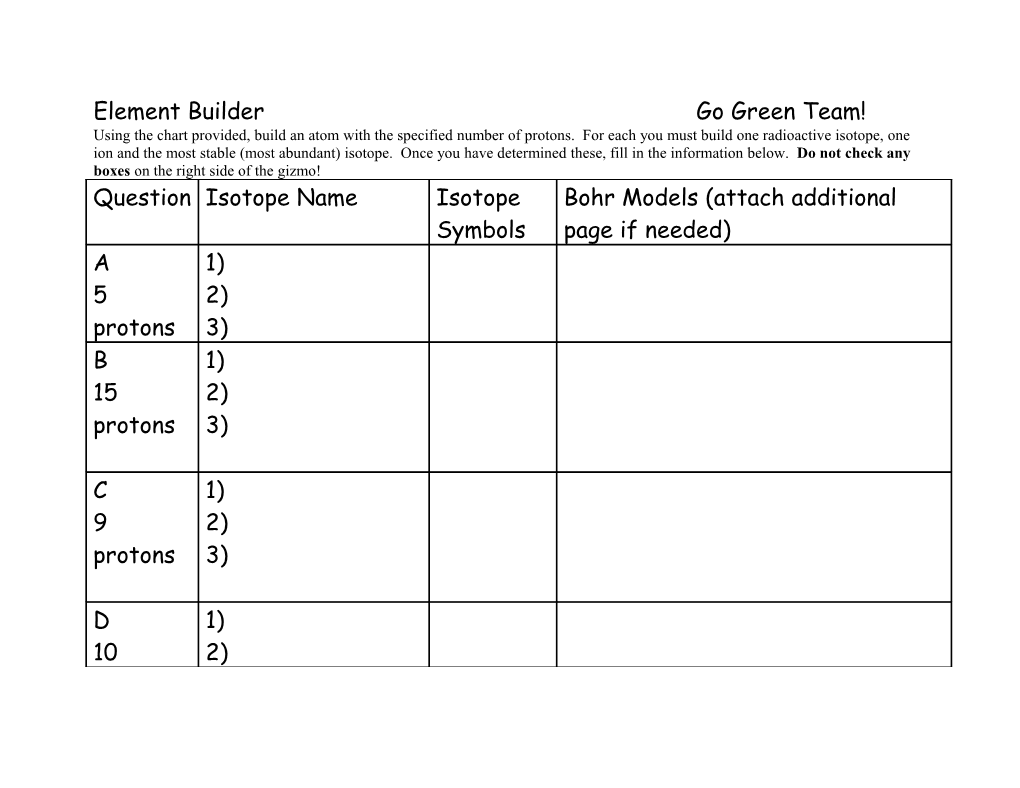
Element Builder Go Green Team! Using the chart provided, build an atom with the specified number of protons. For each you must build one radioactive isotope, one ion and the most stable (most abundant) isotope. Once you have determined these, fill in the information below. Do not check any boxes on the right side of the gizmo! Question Isotope Name Isotope Bohr Models (attach additional Symbols page if needed) A 1) 5 2) protons 3) B 1) 15 2) protons 3)
C 1) 9 2) protons 3)
D 1) 10 2) protons 3)
E 1) 3 2) protons 3)
F 1) 17 2) protons 3)
Questions: Please answer these thoroughly. 1. What causes elements to become ionized? 2. Is there a trend to help predict whether an isotope is radioactive or stable? 3. Draw a graph using the stable isotopes (#3’s above) and plot the number of neutrons on the y-axis vs. number of protons on the x-axis. Determine the slope of this line. What does this slope indicate? 4. Build Carbon-12 and Carbon-13. Use the %abundance information provided to determine the average atomic mass.