SOME TOUGH COLLEGE PROBLEMS!
LEWIS DOT STRUCTURES
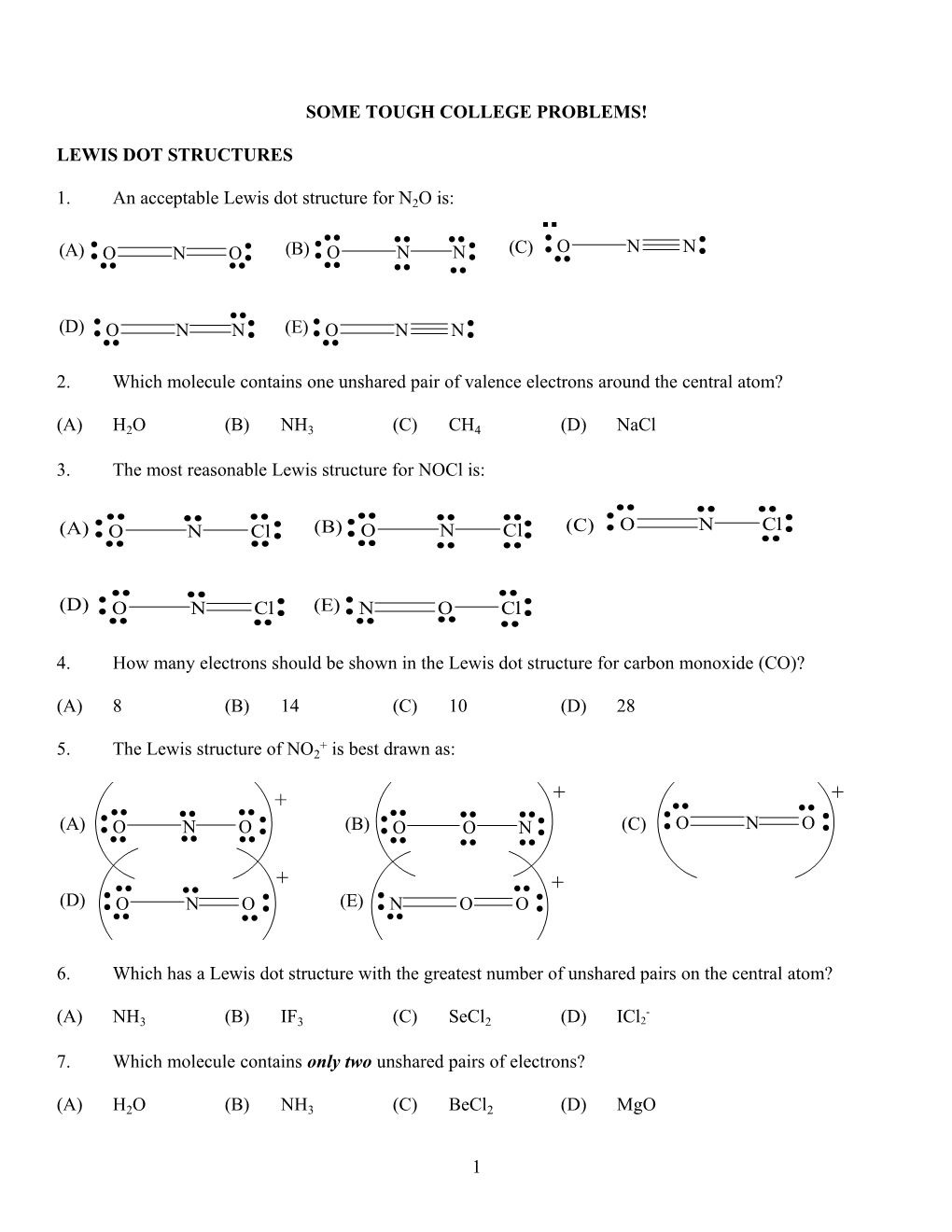
1. An acceptable Lewis dot structure for N2O is: ...... (A) (B) (C) :O N N: :.O. N .O.: :.O. .N. .N.: .. .. (D) :.O. N N: (E):.O. N N:
2. Which molecule contains one unshared pair of valence electrons around the central atom?
(A) H2O (B) NH3 (C) CH4 (D) NaCl
3. The most reasonable Lewis structure for NOCl is: ...... (B) (C) :O N Cl (A) :.O. N .C.l: :.O. .N. .C.l: ..: ...... (D) :.O. N .C.l: (E):.N. .O. .C.l:
4. How many electrons should be shown in the Lewis dot structure for carbon monoxide (CO)?
(A) 8 (B) 14 (C) 10 (D) 28
+ 5. The Lewis structure of NO2 is best drawn as: ...... + ...... + .. .. + (A) :.O. .N. .O.: (B) :.O. .O. .N.: (C) :O N O : .. .. + .. + (D) :.O. N .O.: (E) :.N. O O :
6. Which has a Lewis dot structure with the greatest number of unshared pairs on the central atom?
- (A) NH3 (B) IF3 (C) SeCl2 (D) ICl2
7. Which molecule contains only two unshared pairs of electrons?
(A) H2O (B) NH3 (C) BeCl2 (D) MgO
1 8. Which of the following molecules would have a trigonal planar molecular arrangement?
(A) CBr4 (B) BF3 (C) OF2 (D) PCl3 (E) AsF5
9. In the Lewis structure of SF2, there are ____ single bonds and ____ total unshared pairs?
(A) 2,8 (B) 3,8 (C) 2,2 (D) 2,6 (E) 3,6
10. Which of the following species is INCORRECTLY matched with the number of unshared pairs found on the central atom?
2- - - (A) SO4 0 (B) NO3 1 (C) XeF2 3 (D) SO2 1 (E) IF4 2
11. Which of the following molecules violates the octet rule?
(A) CBr4 (B) NF3 (C) OF2 (D) PCl3 (E) AsF5
12. Which of these contains at least one multiple bond?
-1 -1 (A) NO3 (B) PH3 (C) N2H4 (D) SiCl4 (E) BH4
13. Sulfur dioxide can be described by the structures below. This implies that: ...... O. S .O.: :.O. S .O.
(A) The two bonds in SO2 are of equal length, and the electronic distribution in the two SO bonds is identical, because the two different bonds seem to exchange positions. (B) The single bond is longer than the double bond and the electronic distribution in the two SO bonds is different. (C) This molecule doesn’t exist in any form, due to the fact that the formal charges are constantly changing. (D) The SO2 molecule revolves so that the two different bonds seem to exchange positions. O O -2 14. How many different resonance forms are possible for the oxalate anion, C2O4 ? O C C O (A) 3 (B) 7 (C) 6 (D) 1 (E) 4
15. Which molecule exhibits resonance? F H
(A) O3 (B) BeCl2 (C) CO2 (D) H2Se (E) NF3 F B N H
F H 16. What are the formal charges on the boron and nitrogen in the compound BF3-NH3?
(A) -2 and +2 (B) +2 and –2 (C) 0 and 0 (D) +1 and –1 (E) -1 and +1
2 2 – 17. The structure of the CO3 ion can be described by these Lewis structures which means that: 2- 2- 2- .. .. :O: :O: :O:
C .. .. C .. C .. :.O. .O.: :.O. .O.: :.O. .O.: (A) two CO bonds are single bonds, the third CO bond is a double bond. 2 – (B) these three independent forms of the CO3 ion co-exist in nature. -2 (C) CO3 doesn’t exist in nature at all. 2 – (D) the CO3 ion exists in only one form which is a composite or average of the three structures shown above, because the double bond is rapidly moving among the three forms.
18. Which is the least significant contributing structure to the resonance hybrid of the nitrate ion? - - (A) .. (B) :O: :O:
.. N .. .. N .. :.O. .O.: :.O. .O.:
- - (C) .. (D) .. :O: :O:
N .. .. N :.O. .O.: :.O. .O.:
+ 19. What are the formal charges on each of the atoms in the PH4 ion?
(A) P = 0; H = ¼+ (B) P = 0, H = 0 (C) P = 4+; H = 1- (D) P=1+; H=0 (E)P=0; H=1+
-2 20. What is the formal charge on the sulfur atom in the sulfate anion (SO4 ), drawn without an expanded octet?
(A) -2 (B) 0 (C) +2 (D) +4 (E) +6
3 -1 21. What is the formal charge on the nitrogen atom in the nitrate (NO3 ) anion?
(A) -2 (B) -1 (C) 0 (D) +1 (E) +2
ENERGY IN VS. ENERGY OUT
22. Given the bond energies (in kJ/mol) below, calculate the energy change (in kJ) for
C(g) + H2 (g) + F2 (g) CH2F2(g). Is this a spontaneous process – meaning, is it going to occur easily without much effort?
C—H 414 C—F 486 H—H 435 F—F 159
(A) +900 (B) –900 (C) –465 (D) –1206
23. Calculate energy change for NH3(g) + Cl2(g)NH2Cl(g) + HCl(g)using the bond energies:
N—H 389 Cl—Cl 240 H—Cl 431 N—Cl 201. Is this a spontaneous process – meaning, is it going to occur easily without much effort?
(A) –337 kJ (B) +337 kJ (C) –84 kJ (D) -3 kJ
24. Calculate the energy change for the reaction, C2H4 + H2O C2H5OH using the following bond energies (in kJ/mol). Is this a spontaneous process – meaning, is it going to occur easily without much effort?
C-H 413 C-O 358 C-C 347 O-H 467 H-H 432 C=C 614 C=O 799
(A) -37 (B) +179 (C) -441 (D) -304 (E) +2098
25. Using the bond energies given, estimate the energy change for making carbonic acid (H2CO3) from CO2 and H2O. Is this a spontaneous process – meaning, is it going to occur easily without much effort?
C-O 358 C-C 347 O-H 467 C=O 799
(A) +493 kJ/mol (B) -493 kJ/mol (C) +83 kJ/mol (D) +799 kJ/mol (E) -467 kJ/mol
ELECTRONEGATIVITY AND BOND POLARITY
26. Which would be expected to be the most electronegative?
(A) P (B) As (C) Si (D) Al
27. The element with the greatest tendency to gain electron(s) is:
(A) F (B) At (C) O (D) N (E) Bi
28. Which atom has the highest electronegativity?
(A) Br (B) Mg (C) C (D) O
4 29. Which of these elements should be the most active as a nonmetal, given that their electronegativity values are:
Q 0.9 R 1.0 T 2.8 X 3.0 Z 4.0
(A) Q (B) R (C) T (D) X (E) Z
30. Which of the following statements about electronegativity (EN) is FALSE?
(A) Nonmetals usually have higher EN than metals (B) Differences in electronegativity greater than 1.7 tell us that the bond is non-polar covalent (C) HCl has a higher partial charge than HI because the EN of Cl > EN of I (D) In general, an atom’s EN is inversely related to its radius
31. In which bond are the partial charges on the atoms correct?
(A) +Si-O- (B) +Cl-Br- (C) +N-B- (D) +Cl-Cl-
32. Which of the following bonds are the MOST non-polar?
Atoms H S P As Cl Si Sb Electronegativity 2.1 2.5 2.1 2.1 3.0 1.8 1.9
(A) P-H (B) As-Cl (C) Si-H (D) Sb-Cl (E) H-S
33. Which set of bonds is arranged in order of increasing polarity?
(A) Si-S < Si-O < Si-P < Si-F (B) Si-O < Si-F < Si-S < Si-P (C) Si-F < Si-S < Si-O < Si-P (D) Si-P < Si-S < Si-O < Si-F
34. The IF molecule may be represented by the formula I-F. The polarity is best represented as:
(A) I -F (B) I -F (C) I -F (D) I -F
IONIC BONDING
35. The lattice energy for ionic crystals increases as the charge on the ions ___ and the size of the ions ___.
(A) increases, increases (B) increases, decreases (C) decreases, increases (D) decreases, decreases
36. Select the compound with the highest (most negative) lattice energy?
(A) CaS (B) MgO (C) NaI (D) LiBr
37. Select the compound with the lowest (least negative) lattice energy?
(A) CsBr (B) NaCl (C) SrO (D) CaO
5 38. Calculate the lattice energy for LiBr(s) given the following information:
Li(s) Li(g), H = +166 kJ/mol Hf (LiBr) = -351 kJ/mol Br2(l) 2 Br(g), H = +194 kJ/mol EA of Br(g) = -325 kJ/mol IE of Li(g) = +520. kJ/mol
(A) -906 (B) -1575 (C) -1479 (D) -809 (E) -351
39. Use a Born-Haber cycle based on the formation of MgO to determine heat released for the process, O(g) + 2e O-2(g) in kJ/mol (The total electron affinity)
Mg(s) Mg(g) H = +150 kJ/mol IE1 + IE2 Mg = +2180 kJ/mol Hf (MgO) = -602 kJ/mol Bond energy of O=O = 498 kJ/mol Lattice energy of MgO(s) = -3920 kJ/mol
(A) -1314 (B) +739 (C) +137 (D) +1786 (E) -141
40. Calculate the lattice energy of magnesium sulfide (MgS) given that (in kJ/mol):
Mg(s) Mg(g) H = +153 IE(1) Mg, IE(2) Mg H = +700, +1480 Hf (MgS) H = -343
S (s) S(g) H = +557.5 EA(1) S, EA(2) S H = -100, -203
(A) -6833 kJ/mol (B) -2930.5 kJ/mol (C) 2244 kJ/mol (D) 6147 kJ/mol
41. Calculate the electron affinity of chlorine from the following data (in kJ/mol):
Rb(s) Rb(g) H = 85.8 IE (Rb) H = 397.5 diss E. (Cl2) H =226
H(latt)(RbCl) H = -695 Hf (RbCl) H = -431
(A) -530 kJ/mol (B) -445 kJ/mol (C) -417 kJ/mol (D) -332 kJ/mol
VSEPR
42. Which pairs have the same molecular geometry?
(A) SO2 and CO2 (B) CO2 and OF2 (C) PH3 and BF3 (D) SO2 and O3
+1 43. The molecular geometry for SeF3 is:
(A) trigonal pyramidal. (B) square planar. (C) tetrahedral. (D) rectangular planar.
44. The molecular geometry of BrF5 is: (A) square pyramidal. (B) trigonal pyramidal. (C) trigonal bipyramidal. (D) octahedral.
45. Which has a planar molecular geometry?
-2 -2 +1 (A) NH3 (B) SO3 (C) CO3 (D) H3O
6 46. Which molecule has a linear molecular geometry?
(A) H2O (B) NH3 (C) NO2 (D) CO2 (E) H2S
47. A molecule consists of four bonding pairs of electrons and no lone pairs. What is its molecular geometry?
(A) square planar (B) tetrahedral (C) linear (D) square pyramidal
48. Which statement is true of methane?
(A) It is a tetrahedral molecule. (B) It contains single and double bonds. (C) It has extremely strong chemical bonds (D) It does not occur in nature.
49. Which of the following molecules does NOT possess a trigonal pyramidal molecular geometry?
+1 -1 (A) NH3 (B) COCl2 (C) H3O (D) ClO3 (E) PF3
50. Which of these molecules or ions has a square planar molecular geometry?
+1 - (A) SiBr4 (B) NH4 (C) ClO4 (D) XeF4
51. Which compound would be expected to have charge, or molecular polarity?
(A) SiO2 (B) BCl3 (C) SO2 (D) CF4
52. Which molecule has a permanent molecular polarity or charge?
(A) BCl3 (B) CO2 (C) CF4 (D) H2O
53. The bond type and molecular polarity of SiCl4 are:
Bond Type Polarity of Molecule Bond Type Polarity of Molecule (A) polar nonpolar (B) polar polar (C) nonpolar polar (D) nonpolar nonpolar
54. Which molecule is nonpolar?
(A) CCl4 (B) HCl (C) CF3Cl (D) CHCl3 (E) NH3
55. Experiment shows that the molecule H2Se has a permanent dipole moment, or molecular polarity that is not zero. Which statement MUST therefore be incorrect?
(A) The H2Se molecule is linear. (B) The H2Se molecule is covalent. (C) The H2Se molecule is electrically neutral, or has no charge (D) There must be a difference in electronegativity between hydrogen and selenium.
7 56. Which of the following possesses polar bonds but has no molecular polarity or charge?
(A) BF3 (B) O2 (C) CHCl3 (D) PF3 (E) Cl2
57. Of the molecules CO2, NH3, H2O and CH4, which are polar?
(A) CO2, NH3 and H2O (B) NH3, H2O and CH4 (C) CO2 and CH4 (B) CO2 and NH3 (E) NH3 and H2O
58. Which of the following has charge, or molecular polarity that is not zero?
(A) BBr3 (B) SF2 (C) XeF4 (D) CO2 (E) N2O
59. Which of the following has no molecular polarity or charge?
(A) CS2 (B) H2O (C) CH2F2 (D) PCl3 (E) CH2O
GENERAL STUFF
60. Which one of the following properties is NOT characteristic of substances composed of small, covalently-bonded molecules, with no slight charge?
(A) don’t dissolve in water (B) low boiling point (C) usually solids (D) 2 or more non-metals sharing electrons (E) usually gases
61. Which of the following properties is least characteristic of metals?
(A) high melting point (B) high boiling point (C) brittleness (D) good electrical conductor when solid (E) shiny and lustrous
62. Which of the following properties is least characteristic of ionic compounds?
(A) high melting point (B) high boiling point (C) transfer of electrons (D) usually solids (E) poor electrical conductor when molten
8