Date ______Hr ______Name ______
Molar Volume of a Gas Determined via a Reaction between Magnesium and Hydrochloric Acid
INTRODUCTION: Today you will be mixing magnesium metal (Mg) and hydrochloric acid solution (HCl). The reaction will generate hydrogen gas (H2(g)). The reaction can be written as (you should balance it!): Mg(s) + HCl(aq) MgCl2(aq) + H2(g) This lab will investigate the relationship between the volume of hydrogen gas generated compared to the moles of hydrogen gas generated – in other words you will be finding the liters per mole of hydrogen gas. For all trials, the hydrochloric acid will be in excess (assuming you follow directions!).
MATERIALS: Magnesium metal HCl (1M) 125mL Conical Flask 2-hole Rubber stopper Vernier gas pressure sensor Vernier interface, cables, and PC Electronic Balance Vernier temperature probe 10 mL and 100mL Graduated Cylinders 50mL beaker Plastic syringe Plastic tubing
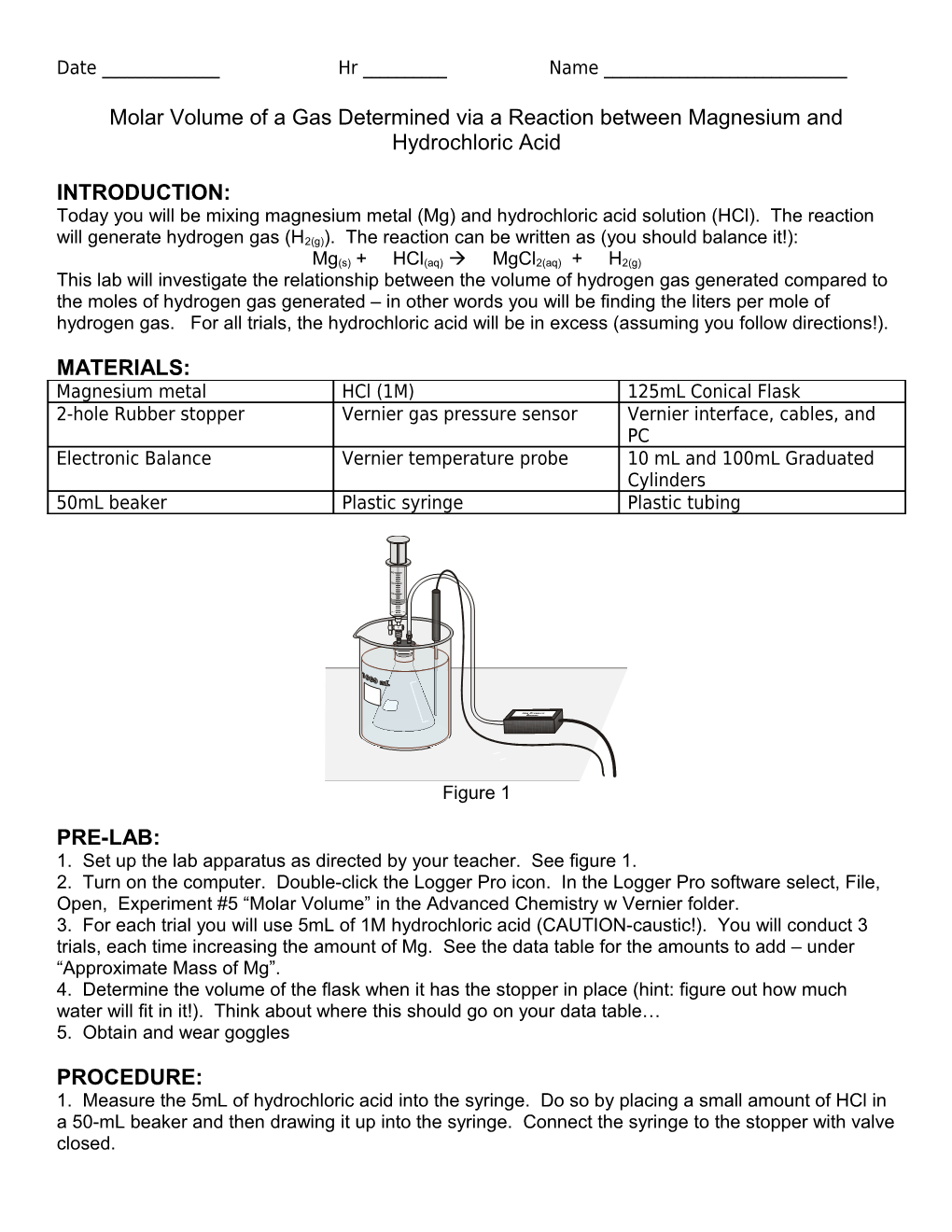
Figure 1
PRE-LAB: 1. Set up the lab apparatus as directed by your teacher. See figure 1. 2. Turn on the computer. Double-click the Logger Pro icon. In the Logger Pro software select, File, Open, Experiment #5 “Molar Volume” in the Advanced Chemistry w Vernier folder. 3. For each trial you will use 5mL of 1M hydrochloric acid (CAUTION-caustic!). You will conduct 3 trials, each time increasing the amount of Mg. See the data table for the amounts to add – under “Approximate Mass of Mg”. 4. Determine the volume of the flask when it has the stopper in place (hint: figure out how much water will fit in it!). Think about where this should go on your data table… 5. Obtain and wear goggles
PROCEDURE: 1. Measure the 5mL of hydrochloric acid into the syringe. Do so by placing a small amount of HCl in a 50-mL beaker and then drawing it up into the syringe. Connect the syringe to the stopper with valve closed. Date ______Hr ______Name ______2. For trial 1, add 0.1g of Mg to the flask. Record the mass to the nearest 0.001g. 3. Prepare a room temperature water bath in a large beaker. The bath should be deep enough to completely cover the gas level in the conical flask. 4. Connect the temperature probe and gas pressure sensors to the Vernier Lab Pro (green box). Be sure the cable is connected properly from the Lab Pro to the PC. 5. Connect the gas pressure sensor to the stopper via the plastic tubing and be sure all connections are snug. 6. In the Logger Pro software make sure that the data-collection rate is set to 1 sample/second and the length is set at 700 seconds. 7. Submerge the flask in the water bath. Press “Collect” to begin data collection. After about 20 seconds, open the valve on the syringe, press the plunger to add all of the 5mL of hydrochloric acid, and pull the plunger back to its original position. Close the two-way valve. 8. Keep the flask immersed in the after bath as the reaction proceeds. Once the maximum pressure is reached you may stop collecting data. 9. Carefully remove the stopper from the flask to relieve the pressure in the flask. DO NOT open the two way valve to release pressure in the flask. 10. Record the temperature, initial pressure, and maximum pressure in the data table before continuing. 11. Repeat for trials 2 and 3. Empty and then rinse the flask with deionized water between trials. 12. When finished put away all equipment in the places indicated by your teacher.
Data Table 1 Trial 1 Trial 2 Trial 3 Approximate Mass of Mg (g) 0.01 0.02 0.03
Actual Mass Mg Used (g)
Moles Mg Used
Moles H2 Generated
Volume H2 Generated (L)
Temp. (K)
Initial Pressure (kPa)
Maximum Pressure (kPa)
Pressure change (kPa)
CALCULATIONS: 1. Calculate the moles of Mg used for each trial and enter it in the data table. 2. Using the balanced equation and stoichiometry, calculate the moles of H2 gas generated. This is a theoretical amount, but for purposes of this lab we’ll assume it’s the same as the actual amount in your flask. Show work for calculations #1-2 for one of your trials in the space below: Date ______Hr ______Name ______
QUESTIONS/ANALYSIS: Molar Volume of a Gas Gases under what we call “ideal” conditions behave the same – they will take up the same volume and exert the same pressure assuming all other things are equal. In essence, it does not matter what type of gas atom or molecule it is. Carbon dioxide (CO2) will behave the same as Hydrogen (H2), which will behave the same as Xenon (Xe). Because gas volumes/pressures change if temperature or pressure changes, chemists use conditions called Standard Temperature and Pressure (STP) as a way of comparing gases. STP is 273K (0°C) and 1.00 atm of pressure. If we always correct to STP then we will always be comparing gases under the same conditions.
3. Before you can determine the molar volume of a gas, we must correct the conditions in our lab to STP. You can use the combined gas law to correct the volume of gas you obtained to STP (P2 and V2 are STP, solve for V2). Your P1, V1, and T1 values are in your Data Table 1 above. Be sure you think carefully about which value is which. P V P V Combined Gas Law: 1 1 = 2 2 T T 1atm = 1 2 101.325kPa Data Table 2 Trial 1 Trial 2 Trial 3
Volume of H2 gas at STP (L) Show your work for at least one of your trials here:
4. Now that you have your volumes corrected to STP you can calculate the Molar Volume of a gas (in other words, the volume of one mole of your gas). To do this, simply divide the volume by the moles for each trial. Data table 3 Trial 1 Trial 2 Trial 3 Average Molar Volume (L/mol) Show your work for at least one of your trials here:
5. The accepted value for the Molar Volume of a gas at STP is 22.4 L/mol. Compare your average Molar Volume to the accepted value by calculating a percent error. Show your work. Date ______Hr ______Name ______
The Ideal Gas Law The ideal gas law relates pressure, volume, moles, and temperature. Another way we can check our lab results is by calculating R, the gas constant, from our lab data. Use your lab data to find your value for the gas constant. PV = nRT or R = (PV)/(nT)
Data Table 4 (you can find the data for this table in previous data tables) Trial 1 Trial 2 Trial 3 Temperature (K)
Volume H2 (L)
Pressure of H2 (kPa)
Moles H2
R (L•kPa/mol•K) Average =
6. The accepted value for R is 8.314 L•kPa/mol•K. Compare your average value for the gas constant to the accepted value by calculating a percent error. Show your work.