Ecological Studies on Hawaiian Lava Tubes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hawaii Volcanoes National Park Geologic Resources Inventory Report
National Park Service U.S. Department of the Interior Natural Resource Program Center Hawai‘i Volcanoes National Park Geologic Resources Inventory Report Natural Resource Report NPS/NRPC/GRD/NRR—2009/163 THIS PAGE: Geologists have lloongng been monimonittoorriing the volcanoes of Hawai‘i Volcanoes National Park. Here lalava cascades durduriingng the 1969-1971 Mauna Ulu eruption of Kīlauea VolVolcano. NotNotee the Mauna Ulu fountountaiain in the background. U.S. Geologiogicalcal SurSurvveyey PhotPhotoo by J. B. Judd (12/30/1969). ON THE COVER: ContContiinuouslnuouslyy eruptuptiingng since 1983, Kīllaueaauea Volcano contcontiinues to shapshapee Hawai‘Hawai‘i VoVollccanoes NatiNationalonal ParkPark.. Photo courtesy Lisa Venture/UniversiUniversitty of Cincinnati. Hawai‘i Volcanoes National Park Geologic Resources Inventory Report Natural Resource Report NPS/NRPC/GRD/NRR—2009/163 Geologic Resources Division Natural Resource Program Center P.O. Box 25287 Denver, Colorado 80225 December 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Denver, Colorado The National Park Service, Natural Resource Program Center publishes a range of reports that address natural resource topics of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate high-priority, current natural resource management information with managerial application. The series targets a general, diverse audience, and may contain NPS policy considerations or address sensitive issues of management applicability. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. -

The Cavernicolous Fauna of Hawaiian Lava Tubes, 1
Pacific Insects 15 (1): 139-151 20 May 1973 THE CAVERNICOLOUS FAUNA OF HAWAIIAN LAVA TUBES, 1. INTRODUCTION By Francis G. Howarth2 Abstract: The Hawaiian Islands offer great potential for evolutionary research. The discovery of specialized cavernicoles among the adaptively radiating fauna adds to that potential. About 50 lava tubes and a few other types of caves on 4 islands have been investigated. Tree roots, both living and dead, are the main energy source in the caves. Some organic material percolates into the cave through cracks associated with the roots. Cave slimes and accidentals also supply some nutrients. Lava tubes form almost exclusively in pahoehoe basalt, usually by the crusting over of lava rivers. However, the formation can be quite complex. Young basalt has numerous avenues such as vesicles, fissures, layers, and smaller tubes which allow some intercave and interlava flow dispersal of cavernicoles. In older flows these avenues are plugged by siltation or blocked or cut by erosion. The Hawaiian Islands are a string of oceanic volcanic islands stretching more than 2500 km across the mid-Pacific. The western islands are old eroded mountains which are now raised coral reefs and shoals. The eight main eastern islands total 16,667 km2 and are relatively young in geologic age. Ages range from 5+ million years for the island of Kauai to 1 million years for the largest island, Hawaii (Macdonald & Abbott, 1970). The native fauna and flora are composed of those groups which dis persed across upwards of 4000 km of open ocean or island hopped and became successfully established. -

America's Natural Nuclear Bunkers
America’s Natural Nuclear Bunkers 1 America’s Natural Nuclear Bunkers Table of Contents Introduction ......................................................................................................... 10 Alabama .............................................................................................................. 12 Alabama Caves .................................................................................................. 13 Alabama Mines ................................................................................................. 16 Alabama Tunnels .............................................................................................. 16 Alaska ................................................................................................................. 18 Alaska Caves ..................................................................................................... 19 Alaska Mines ............................................................................................... 19 Arizona ............................................................................................................... 24 Arizona Caves ................................................................................................... 25 Arizona Mines ................................................................................................... 26 Arkansas ............................................................................................................ 28 Arkansas Caves ................................................................................................ -

Cave Science News
CAV E SCIENCE NEWS CAVE SCIENCE NEWS KARST MEETING PLANNED AT MAMMOTH CAVE Kempe, S. (1996) Enlargement of lava tubes by downcutting and breakdown. Abstracts and Proceedings of the National A joint meeting of the International Geological Correlation Speleological Society Convention 58(3): 203. Program, Project 379: “Karst Processes and the Global Kempe, S. (1996). Neue Rekorde in Lavahohlen auf Hawaii, Carbon Cycle” along with Friends of Karst will take place on ein Statusbericht. Mitt. Arge f. Karstkunde Harz e.V. September 23, 24, and 25, 1998, at Mammoth Cave, Kentucky, 1996(3): 46-49 und (mit gleichem Text) Lavahohlen auf USA. The meeting will be hosted by the Center for Cave and Hawaii ein Statusbericht. Mitt.Verb. Dt. Hohlen- u. Karst Studies at Western Kentucky University, Mammoth Cave Karstforscher 42 (2): 27-29. (German) National Park, and the Cave Research Foundation. Kempe, S. (in press). Lava Falls: a major factor for the Preliminary plans are to have two days of scientific pre- enlargement of lava tube of the Ai-laau shield phase, sentations on various aspects of karst science, with particular Kilauea, Hawaii. Proceedings of the 10th International sessions so far anticipated on progress in understanding the Congress of Speleology. Switzerland. impact of karst processes on carbon cycling at a variety of Kempe, S., Buchas, H., Hartmann, J., Oberwinder, M., scales, and on recent work in the Central Kentucky Karst. A Strassenburg, J. & Wolniewicz, K. (in press). Mapping third day will be planned for a variety of surface and subsur- lava flows by following their tubes: The Keauhou face field trips in and around the Mammoth Cave System, Trail/Ainahou Ranch Flow Field, Kilauea, Hawaii. -

NEWSLETTERS NUMBER 01 to 06 (5.7
REDUCED-SIZE PHOTOCOPIES of: Newsletter # 1 .... 6 _,_--~~ ... ... "" "- ~ ,___,. ..\ ..' . 4 .. ~ COMMISSION on VOLCANIC CAVES . COMM I SSION ON VOLCANIC CAVES International Union of Speleology Newsletter #l December 22, 199~ STATUS OF IUS WORKING GROUP ON VOLCANIC CAVES RAISED TO COMMISSION The Working Group on Volcanic Caves of the International Union of Speleology h as been elevated to full Comm ission s tatus. By letter of 13 December 1993, new IUS President Paulo Forti confirmed that the IUS Bureau took the necessary action during the international congress of speleology in Beijing earlier this year. The membership of t he new commission is the same as the membership of the old working group, with one notable recent appointment, a nnounce d on this page. Names and addresses of t h e members a r e elsewhere in this issue. Because of t he change in name and status , this is Newsletter #1 of t he new commission, rather t han Newsletter #6 . Th e next issue is expected to be in Spring, 1994. Items for publication in it should be sent to the chairman at h is Hawaii address before March 1. DR. YURII B. SLEZ IN APPOINTED TO COMMISSION Yuri i B. Slezin h as accepted sppointment to t he commission. He has studied l ava flows in tubes of the 1975-1976 Tolbachic eruption on Kamchatka and the structure of a similar o l d lava field nea rby. In this lava field he mapped a cave which is a fragment of a great lava tube system. Other fragments of the system, inc l uding caves about 100-150 meters long, are present, and other tube systems in a dditidn~ This is the only known published map of a lava t ube cave i n Russia. -

The Cavernicolous Fauna of Hawaiian Lava Tubes, Part Vi
Technical Report No. 40 THE CAVERNICOLOUS FAUNA OF HAWAIIAN LAVA TUBES, PART VI. MESOVELIIDAE OR WATER TREADERS (HETEROPTERA) , Wayne c. Gagne and Francis G. Howarth Department of Entomology Bernice P. Bishop Museum Honolulu, Hawaii 96818 ISLAND ECOSYSTEMS IRP U. s. International Biological Program May• 1974 ABSTRACT This paper is a systematic report and a biological analysis of the Mesoveliidae from Hawaiian caves. Most important is a new species of Speovelia Esaki, ~. sp. (Hawaii I.), described from material collected on moist walls in lava tubes. This apparently represents the world's first troglobitic heteropteran. Morphologically, all instars show lack of pigmentation, functionless eyes and prolongation of the appendages and head. Biologically, it is restricted to the dark zone of lava tubes. Notes on its biology are given. It also represents the first known endemic species of the family for the Hawaiian Islands. Another species, Mesovelia amoena Uhler, is troglophilic. This is the first record of this immigrant American species in the Hawaiian Islands. The remaining species in Hawaii, the immigrant'M· mulsanti White, has only been found in epigean situations. A key to the species is also presented. - i - TABLE OF CONTENTS Page ABSTRACT i INTRODUCTION 1 KEY TO HAWAIIAN MESOVELIIDAE 2 S PEOVELIA SP. 3 Taxonomic description 3 Distribution 5 Etymology and Remarks 6 Biology 7 Habitat 7 Food web 9 Associated biota 9 Distribution within caves 10 Population 10 Behavior 11 Food habits • 11 Comparative feeding and reproductive habits 12 SPEOVELIA MARITIMA ESAKI 13 MESOVELIA AMOENA UHLER 13 Taxonomic description • 13 Material examined 15 Remarks 15 MESOVELIA MULSANTI WHITE • 16 MESOVELIA SP. -

Journal of Cave and Karst Studies Editor Louise D
December 1998 JOURNAL OF Volume 60 Number 3 ISSN 1090-6924 CAVE AND KARST STUDIES Journal of Cave and Karst Studies Editor Louise D. Hose Volume 60 Number 3 December 1998 Environmental Studies Program Westminster College CONTENTS Fulton, MO 65251-1299 (573) 573-5303 Voice (573) 592-2217 FAX Articles [email protected] Tubular Lava Stalactites and Other Related Segregations Production Editor Kevin Allred and Carlene Allred 131 James A. Pisarowicz Wind Cave National Park Hot Springs, SD 57747 History and Status of the Moiliili Karst, Hawaii (605) 673-5582 William R. Halliday 141 [email protected] Gypsum Speleothems of Freezing Origin BOARD OF EDITORS Victor V. Korshunov and Elena V. Shavrina 146 Earth Sciences-Journal Index Ira D. Sasowsky Geochemistry of Fluorite and Related Features of the Department of Geology University of Akron Kugitangtou Ridge Caves, Turkmenistan Akron, OH 44325-4101 Vladimar Maltsev and Viktor Korshunov 151 (330) 972-5389 [email protected] The Cave-inhabiting Beetles of Cuba (Insecta: Coleoptera): Diversity, Distribution and Ecology Conservation Stewart B. Peck, Amador E. Ruiz-Baliú and George Huppert Department of Geography Gabriel F. Garcés González 156 University of Wisconsin, LaCrosse LaCrosse, WI 54601 Spatial and Temporal Variations in the Dissolved Organic [email protected] Carbon Concentrations in the Vadose Karst Waters of Marengo Cave, Indiana Life Sciences Veronica A. Toth 167 David Ashley Department of Biology Missouri Western State College The Current Status and Habitats of the Illinois Cave St. Joseph, MO 64507 Amphipod, Gammarus acherondytes Hubricht and (816) 271-4334 Mackin (Crustacea: Amphipoda) [email protected] D.W. -

The Texas Caver
the Texas Caver OCTOBER 2004 the Texas Caver October 2004 CONTENTS Volume 50 Number 5 Caving Events 3 ISSN 0040-4233 Photo Caption Contest 3 the Texas Caver is a bi-monthly publication of The Texas Speleological Association (TSA), an internal organization of the Punkin & Deep Purchase 4 National Speleological Society (NSS). Rescue in Matacanes! 5 Subscription rates are $20/year which includes TSA member- ship. Libraries, institutions, and out-of-state subscribers NSS Convention 13 may receive The Texas Caver for $20/year. Student subscriptions are $15/year. Cave Ballads 14 Letters to the Editor, article and photo submissions, advertising and questions should be sent to the Editor: Old Timer’s Reunion 15 the Texas Caver From the Files of the TSS: the c/o Diana Tomchick 10106 Technology Blvd. West Franklin Mountains Paleokarst 16 Apt. #826 Dallas, TX 75220 Trip Reports: Honey Creek 18 [email protected] (214) 418-5827 Trip Reports: Station C Cave 19 The deadline for submissions to the Texas Caver is the 7th day of odd-numbered months. Trip Reports: Tyra’s Cave 21 Opinions expressed in the Texas Caver are solely those of the Southwest Labor Day Regional, authors and do not necessarily reflect the views of the editors, the TSA or the NSS. North Percha Creek 22 Subscription requests, newsletter exchanges and address Book Reviews 24 changes should be sent to the Texas Speleological Association Business Address: Punkin & Deep Interim The Texas Speleological Association P. O. Box 8026 Management Plan 25 Austin, TX 78713 TCR 27 Texas -

Hawaii Volcanoes National Park Geologic Resources
National Park Service U.S. Department of the Interior Natural Resource Program Center Hawai‘i Volcanoes National Park Geologic Resources Inventory Report Natural Resource Report NPS/NRPC/GRD/NRR—2009/163 THIS PAGE: Geologists have long been monitoring the volcanoes of Hawai‘i Volcanoes National Park.k. Here lava cascades during the 1969-1971 Mauna Ulu eruption of Kīlauea Vollcano. Note the Mauna Ullu fountain in tthee background. U.S. Geological Survey Photo by J. B. Judd (12/30/1969). ON THE COVER: Continuously erupting since 1983, Kīllaueaauea Vollcanocano continues to shape Hawai‘i Volcanoes National Park. Photo courtesy Lisa Venture/University of Cincinnati. Hawai‘i Volcanoes National Park Geologic Resources Inventory Report Natural Resource Report NPS/NRPC/GRD/NRR—2009/163 Geologic Resources Division Natural Resource Program Center P.O. Box 25287 Denver, Colorado 80225 December 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Denver, Colorado The National Park Service, Natural Resource Program Center publishes a range of reports that address natural resource topics of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Report Series is used to disseminate high-priority, current natural resource management information with managerial application. The series targets a general, diverse audience, and may contain NPS policy considerations or address sensitive issues of management applicability. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. -

Journal of Cave and Karst Studies Editor Louise D
August 1997 Volume 59 Number 2 JOURNAL OF ISSN 1090-6924 The CAVE AND KARST National Speleological Society STUDIES Bulletin THIS ISSUE: Development and Morphology of Kazumura Cave, A Model of Structure and Genesis for the Gypsum Hawaii “Nest”, Found in the Geophysichejkaya Cave (Kugitangtou Mountains, Turkmenistan) Initial Geologic Observations in Caves Bordering the Sibari Plain (Southern Italy) Examining Early Nineteenth Century Saltpeter Caves: An Archaeological Perspective Journal of Cave and Karst Studies Editor Louise D. Hose Volume 59 Number 2 August 1997 Environmental Studies Program Westminster College CONTENTS Fulton, MO 65251-1299 (573) 592-1303 Voice (573) 642-2175 FAX Articles [email protected] Production Editor Development and Morphology of Kazumura Cave, Hawaii James A. Pisarowicz Kevin Allred and Carlene Allred 67 Wind Cave National Park Hot Springs, SD 57747 (605) 673-5582 Initial Geologic Observations in Caves Bordering the [email protected] Sibari Plain (Southern Italy) Sandro Galdenzi 81 BOARD OF EDITORS Earth Sciences-Journal Index A Model of Structure and Genesis for the Gypsum “Nest”, Ira D. Sasowsky Department of Geology Found in the Geophysicheskaya Cave (Kugitangtou Mountains, University of Akron Turkmenistan) Akron, OH 44325-4101 (330) 972-5389 V.A. Maltsev 87 [email protected] Examining Early Nineteenth Century Saltpeter Caves: Conservation An Archaeological Perspective George Huppert Department of Geography M. Susan Duncan 91 University of Wisconsin, LaCrosse LaCrosse, WI 54601 Cave Science News 95 [email protected] Life Sciences Guide to Authors 96 David Ashley Department of Biology Missouri Western State College St. Joseph, MO 64507 (816) 271-4334 [email protected] Social Sciences Marion O. -
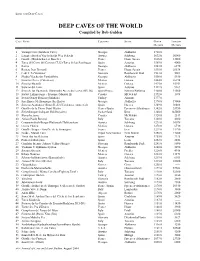
Long and Deep Caves of the World
LONG AND DEEP CAVES DEEP CAVES OF THE WORLD Compiled by Bob Gulden CAVE NAME COUNTRY STATE DEPTH LENGTH METERS METERS 1 Voronja Cave (Krubera Cave) Georgia Abkhazia 1710.0 ----- 2 Lamprechtsofen Vogelschacht Weg Schacht Austria Salzburg 1632.0 50000 3 Gouffre Mirolda/Lucien Bouclier France Haute Savoie 1626.0 13000 4 Torca del Cerro del Cuevon (T.33)-Torca de las Saxifragas Spain Asturias 1589.0 4000 5 Sarma Georgia Abkhazia1543.0 6370 6 Reseau Jean Bernard France Haute Savoie 1535.0 20536 7 Cehi 2 “la Vendetta” Slovenia Rombonski Podi 1533.0 5061 8 Shakta Vjacheslav Pantjukhina Georgia Abkhazia 1508.0 5530 9 Sistema Cheve (Cuicateco) Mexico Oaxaca 1484.0 26194 10 Sistema Huautla Mexico Oaxaca 1475.0 55953 11 Sistema del Trave Spain Asturias 1441.0 9167 12 Sima de las Puertas de Illaminako Ateeneko Leizea (BU.56) Spain/France Navarra/Nafarroa 1408.0 14500 13 Sustav Lukina jama - Trojama (Manual II) Croatia Mt.Velebit 1392.0 1078 14 Evren Gunay Dudeni (Sinkhole) Turkey Anamur 1377.0 ----- 15 Sniezhnaja-Mezhonnogo (Snezhaya) Georgia Abkhazia 1370.0 19000 16 Sistema Aranonera (Sima S1-S2)(Tendenera connected) Spain Huesca 1349.0 36468 17 Gouffre de la Pierre Saint Martin France/Spain Pyrenees-Atlantiques 1342.0 53950 18 Siebenhengste-hohgant Hohlensystem Switzerland Bern 1340.0 145000 19 Slovacka jama Croatia Mt.Velebit 1320.0 2519 20 Abisso Paolo Roversi Italy Toscana 1300.0 4000 21 Cosanostraloch-Berger-Platteneck Hohlesystem Austria Salzburg 1291.0 30076 22 Cueva Charco Mexico Oaxaca 1278.0 6710 23 Gouffre Berger - Gouffre de la Fromagere France Isere 1271.0 31790 24 Neide - Muruk Cave Papua New Guinea New Britain 1258.0 17000 25 Torca dos los Rebecos Spain Asturias 1255.0 2228 26 Pozo del Madejuno Spain Leon 1252.0 2852 27 Crnelsko brezno (Abisso Veliko Sbrego) Slovenia Rombonaki Podi 1241.0 8090 28 Vladimir V. -

NARACOORTE CAVES NATIONAL PARK: VISITOR FACILITY UPGRADES Deborah Carden
Journal of the Australasian Cave and Karst Management Association No. 105 December 2016 Australia Post- Print Post Approved: PP381624/02050. ISSN 1835-5374 Published Quarterly The ACKMA Journal ACKMA Inc. OFFICE BEARERS 2016-17 Official Publication of the Australasian Cave and President Karst Management Association Incorporated Dale Calnin Email: [email protected] Published quarterly in March, June, September and New Zealand Vice President December Neil Collinson Email: [email protected] The opinions expressed in the ACKMA Journal are those of the individual authors and not necessarily Australian Vice President those of ACKMA Inc. or its officers. John Brush Email: [email protected] EDITOR: Steve Bourne Executive Officer SUB EDITORS: Tony Culberg, Andy Spate Dan Cove Email: [email protected] Photos taken by the authors or editor unless Treasurer and Membership Officer otherwise acknowledged. Deborah Carden Email: [email protected] PRINTER: Hansen Print, Smith Street, Naracoorte, South Australia 5271. Ph: (08) Publications Officer and ASF Liaison Officer 87623699 Steve Bourne Email: [email protected] ACKMA Inc. is cross affiliated or otherwise Committee member associated with: Scott Melton Email: [email protected] Australian Speleological Federation, New Zealand Speleological Society, Australasian Bat Society, Committee Member The WCPA Working Group on Cave and Karst Tim Moulds Email: [email protected] Protection, Guiding Organisations Australia, Bat Conservation International, American Cave Committee Member Conservation Association, International Show Ann Augusteyn Email: [email protected] Caves Association, Cave Diving Association of Australia, The Malaysian Karst Society, The Webmaster Jenolan Caves Historical & Preservation Society Rauleigh Webb Email: [email protected] and the USA National Speleological Society Cave Conservation and Management Section Public Officer LIFE MEMBERS of ACKMA Inc.