1
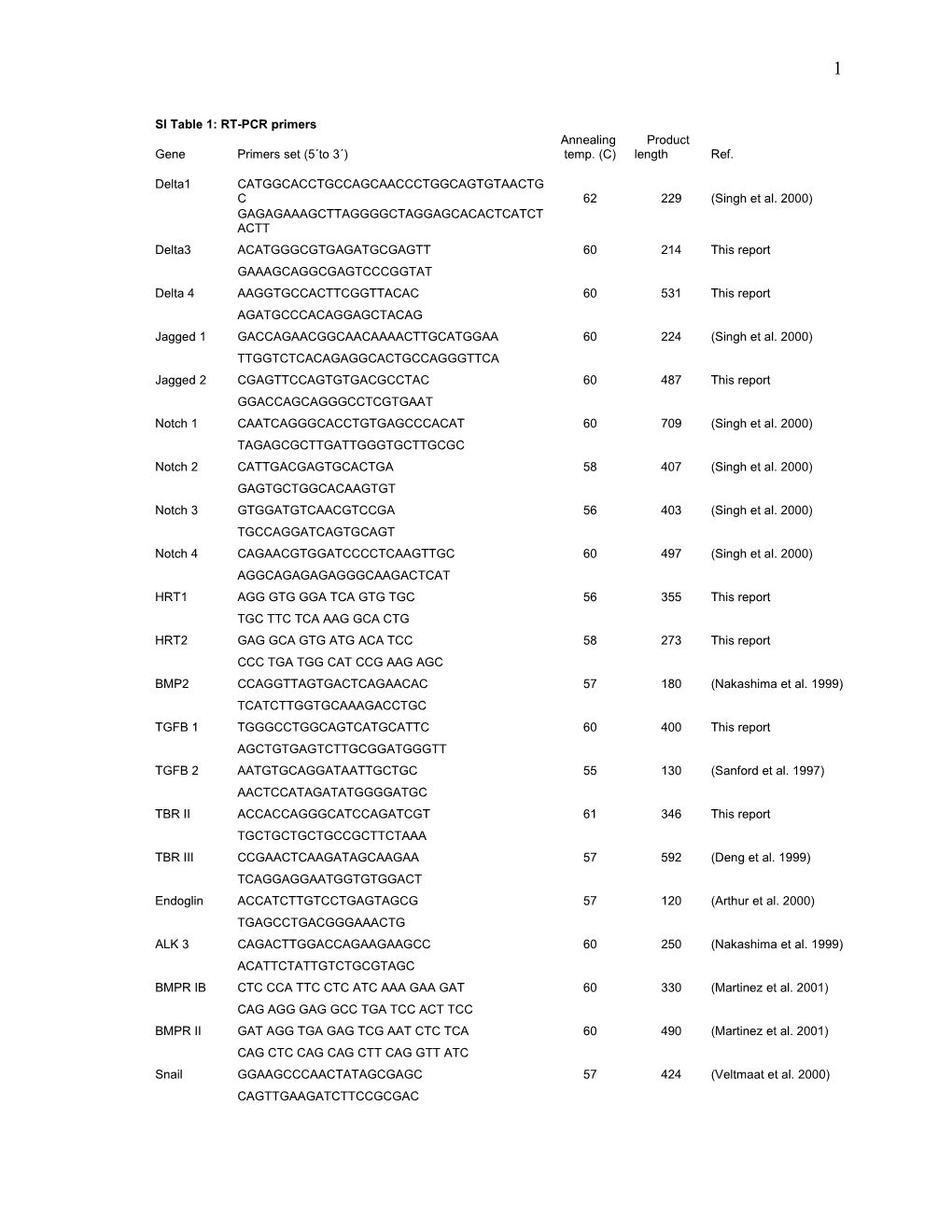
SI Table 1: RT-PCR primers Annealing Product Gene Primers set (5´to 3´) temp. (C) length Ref.
Delta1 CATGGCACCTGCCAGCAACCCTGGCAGTGTAACTG C 62 229 (Singh et al. 2000) GAGAGAAAGCTTAGGGGCTAGGAGCACACTCATCT ACTT Delta3 ACATGGGCGTGAGATGCGAGTT 60 214 This report GAAAGCAGGCGAGTCCCGGTAT Delta 4 AAGGTGCCACTTCGGTTACAC 60 531 This report AGATGCCCACAGGAGCTACAG Jagged 1 GACCAGAACGGCAACAAAACTTGCATGGAA 60 224 (Singh et al. 2000) TTGGTCTCACAGAGGCACTGCCAGGGTTCA Jagged 2 CGAGTTCCAGTGTGACGCCTAC 60 487 This report GGACCAGCAGGGCCTCGTGAAT Notch 1 CAATCAGGGCACCTGTGAGCCCACAT 60 709 (Singh et al. 2000) TAGAGCGCTTGATTGGGTGCTTGCGC Notch 2 CATTGACGAGTGCACTGA 58 407 (Singh et al. 2000) GAGTGCTGGCACAAGTGT Notch 3 GTGGATGTCAACGTCCGA 56 403 (Singh et al. 2000) TGCCAGGATCAGTGCAGT Notch 4 CAGAACGTGGATCCCCTCAAGTTGC 60 497 (Singh et al. 2000) AGGCAGAGAGAGGGCAAGACTCAT HRT1 AGG GTG GGA TCA GTG TGC 56 355 This report TGC TTC TCA AAG GCA CTG HRT2 GAG GCA GTG ATG ACA TCC 58 273 This report CCC TGA TGG CAT CCG AAG AGC BMP2 CCAGGTTAGTGACTCAGAACAC 57 180 (Nakashima et al. 1999) TCATCTTGGTGCAAAGACCTGC TGFB 1 TGGGCCTGGCAGTCATGCATTC 60 400 This report AGCTGTGAGTCTTGCGGATGGGTT TGFB 2 AATGTGCAGGATAATTGCTGC 55 130 (Sanford et al. 1997) AACTCCATAGATATGGGGATGC TBR II ACCACCAGGGCATCCAGATCGT 61 346 This report TGCTGCTGCTGCCGCTTCTAAA TBR III CCGAACTCAAGATAGCAAGAA 57 592 (Deng et al. 1999) TCAGGAGGAATGGTGTGGACT Endoglin ACCATCTTGTCCTGAGTAGCG 57 120 (Arthur et al. 2000) TGAGCCTGACGGGAAACTG ALK 3 CAGACTTGGACCAGAAGAAGCC 60 250 (Nakashima et al. 1999) ACATTCTATTGTCTGCGTAGC BMPR IB CTC CCA TTC CTC ATC AAA GAA GAT 60 330 (Martinez et al. 2001) CAG AGG GAG GCC TGA TCC ACT TCC BMPR II GAT AGG TGA GAG TCG AAT CTC TCA 60 490 (Martinez et al. 2001) CAG CTC CAG CAG CTT CAG GTT ATC Snail GGAAGCCCAACTATAGCGAGC 57 424 (Veltmaat et al. 2000) CAGTTGAAGATCTTCCGCGAC 2
VE-C CCTGACTGGAACCAGCACGCT 60 491 This report GTGTGTCGTATGGGGGGCCAC B-Actin GGACCTGGCTGGCCGGGACC 62 583 (Bi et al. 1999) GCGGTGCACGATGGAGGGGC DegSnail-F1:ATG CCI (A/C)GI (A/T)(C/G)IT T(C/T) pSnail (C/T) TIG TIA A(A/G) 50 673 (Locascio et al. 2002) DegSnail-R:GCI C(G/T)I A(A/G)(A/G) TTI (C/G)(A/T)I C(G/T) (A/G) TCI GC(A/G)AAA GC NestedSnail-F: ATG CCI (A/C)GI (A/T)(G/C)I TT(C/T) TIG T 53 621 NestedSnail-R: AIG G(T/C)T T(T/C)T CIC CIG T(A/G) TGI GT pVE-C AACATCACAGTCAAGCACGGGTAT 53 353 This report TCGCCGCCGCCCTCCTCATCGTAG pGAPDH TGATGACATCAAGAAGGTGGTGAAG 53 250 (Conklin et al. 2002) TCCTTGGAGGCCATGTGGACCAT
References
Arthur, H.M., Ure, J., Smith, A.J., Renforth, G., Wilson, D.I., Torsney, E., Charlton, R., Parums, D.V., Jowett, T., Marchuk, D.A. et al. 2000. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol 217: 42-53. Bi, W., Drake, C.J., and Schwarz, J.J. 1999. The transcription factor MEF2C-null mouse exhibits complex vascular malformations and reduced cardiac expression of angiopoietin 1 and VEGF. Dev Biol 211: 255-67. Conklin, B.S., Zhong, D.S., Zhao, W., Lin, P.H., and Chen, C. 2002. Shear stress regulates occludin and VEGF expression in porcine arterial endothelial cells. J Surg Res 102: 13-21. Deng, X., Bellis, S., Yan, Z., and Friedman, E. 1999. Differential responsiveness to autocrine and exogenous transforming growth factor (TGF) beta1 in cells with nonfunctional TGF-beta receptor type III. Cell Growth Differ 10: 11-8. Locascio, A., Manzanares, M., Blanco, M.J., and Nieto, M.A. 2002. Modularity and reshuffling of Snail and Slug expression during vertebrate evolution. Proc Natl Acad Sci U S A 99: 16841-6. Martinez, G., Loveland, K.L., Clark, A.T., Dziadek, M., and Bertram, J.F. 2001. Expression of bone morphogenetic protein receptors in the developing mouse metanephros. Exp Nephrol 9: 372-9. Nakashima, K., Yanagisawa, M., Arakawa, H., and Taga, T. 1999. Astrocyte differentiation mediated by LIF in cooperation with BMP2. FEBS Lett 457: 43-6. Sanford, L.P., Ormsby, I., Gittenberger-de Groot, A.C., Sariola, H., Friedman, R., Boivin, G.P., Cardell, E.L., and Doetschman, T. 1997. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development 124: 2659-70. Singh, N., Phillips, R.A., Iscove, N.N., and Egan, S.E. 2000. Expression of notch receptors, notch ligands, and fringe genes in hematopoiesis. Exp Hematol 28: 527-34. 3
Veltmaat, J.M., Orelio, C.C., Ward-Van Oostwaard, D., Van Rooijen, M.A., Mummery, C.L., and Defize, L.H. 2000. Snail is an immediate early target gene of parathyroid hormone related peptide signaling in parietal endoderm formation. Int J Dev Biol 44: 297-307.
Supplementary Table 2
Expression Developmental stage Total % pattern 24 hpf 36 hpf 48 hpf 56 hpf 72 hpf* 1 22 9 25 10 6 72 (33) 2 10 9 15 14 10 58 (27) 3 8 4 11 6 3 32 (15) 4 9 7 12 10 18 56 (26) Total 49 29 63 40 37 218 (100)
Number of embryos expressing the injected mouse N1IC in the different patterns considered. Expression patterns were categorized as follows: 1, patchy expression throughout the embryo, excluding the heart region; 2, patchy expression throughout the embryo, including the heart region; 3, expression restricted to the heart region; 4, no expression detected. The percentage of each expression pattern is given between brackets. * The expression at 72 hpf, although detectable, was always weaker than the ones found at earlier developmental stages.
SI Movies 1-3. Real-time video recordings of beating heart in 4 days post- fertilization mlc2a-eGFP transgenic embryos. Movie 1 is an untreated embryo. Movie 2 shows a typical N1IC-injected embryo; note the pericardial distension and enhanced valve reinforcement during ventricular systole. Movie 3 shows a typical embryo treated with 100 M DAPT; note the failure to close the atri-ventricular valve and the regurgitation of blood into the atrium during ventricular systole.
SI Figure 1. Overexpression of an activated Notch allele induces large, invasive tumors in PAE xenografts. H+E stained sections of representative xenografts 48 days post transplantation. (A-C) PAE-GFP; (D-F) PAE-N1IC. (A,D) 5X magnification, (B-F) 40X magnification. PA-N1IC xenografts spread through the co-injected Matrigel carrier solution much more readily, compare (F) versus (C), and penetrate further into adjacent mouse muscle tissue (E) versus (B) than PAE- GFP xenografts. PAE-N1IC xenografts were on average 7X larger (volume) than 4
PAE-GFP xenografts. Since much of the PAE-GFP tumor volume is composed of empty matrigel, this under-reports the true graft size differential.
SI Figure 2. N1IC xenografts have normal -catenin expression levels and subcellular localization. (A) PAE-N1IC and PAE-GFP clones express similar amounts of -catenin. (B-D) PAE-N1IC#1 xenograft. (B) DAPI staining, (C) - catenin staining. (D) Overlay showing cytoplasmic localization of -catenin.
Xenografts, Western and stainings Thirty two nude mice (Charles River) were transplanted with 107PAE-GFP or PAE- N1IC cells in 200l media:Matrigel (50:50). Mice were observed daily, mouse weigh and transplant size recorded weekly. Animals were sacrificed at 32 and 48 days post stransplantation, tissue samples were halved and preserved in buffered formalin or OCT. Hematoxylin and eosin staining was performed by standard methods on xenograft tissue. For immunostaining, 5 m frozen sections were fixed in ice cold MEOH:Acetone for 10 minutes, air dried, rehydrated in PBS and stained with a rabbit polyclonal to beta catenin (Sigma C2206) at 1:2000 overnight. Secondary anti Rabbit -488 (1:300 Molecular Probes) and DAPI counter stain. For Western blotting: 60 g of whole cell lysate was resolved on 4-20% SDS PAGE, blotted onto PVDF membrane, blocked PBS-tween 20/5%nonfat dry milk, and probed with a rabbit polyclonal anti beta catenin (Sigma C2206). Anti rabbit-HRP secondary (1:1000), and ECL were used to detect the specific immunoreactive bands.