SUPPORTING INFORMATION
Oxygen reduction on structurally well defined, bimetallic PtRu surfaces: Monolayer
PtxRu1-x/Ru(0001) surface alloys vs. Pt film covered Ru(0001)
S. Brimaud, A.K. Engstfeld, O.B. Alves, H.E. Hoster, and R.J. Behm
Institute of Surface Chemistry and Catalysis, Ulm University, D-89069 Ulm, Germany
1. Electrochemical characterisation of the PtRu bimetallic surfaces
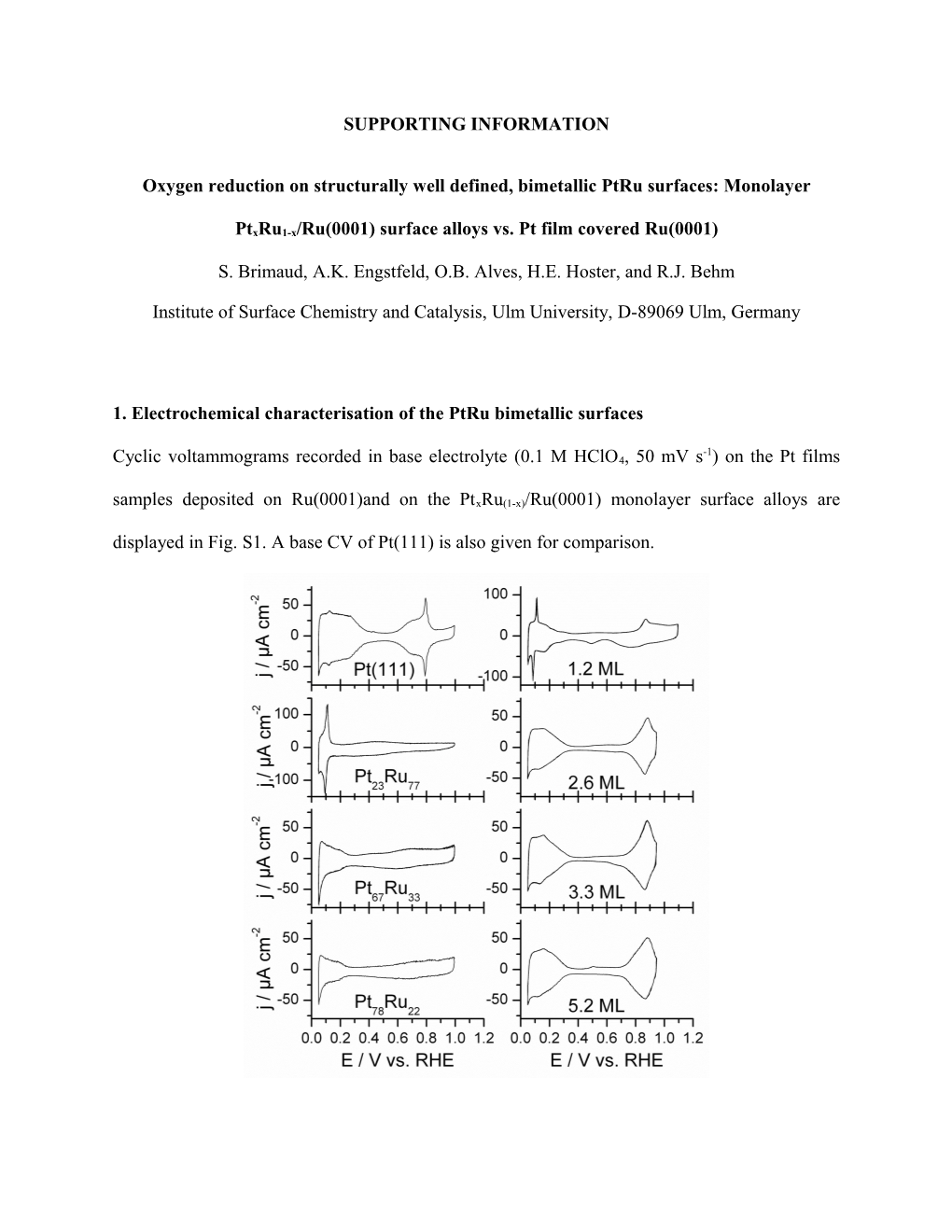
-1 Cyclic voltammograms recorded in base electrolyte (0.1 M HClO4, 50 mV s ) on the Pt films samples deposited on Ru(0001)and on the PtxRu(1-x)/Ru(0001) monolayer surface alloys are displayed in Fig. S1. A base CV of Pt(111) is also given for comparison. Fig. S1 Base cyclic voltammograms for the structurally well-defined PtRu bimetallic surfaces
-1 investigated (0.1 M HClO4, 50 mV s ). 2. Calculated kinetic current densities – Influence of interpolated energies
Fig. S2 compares the experimental kinetic current density for the ORR on Pt(111) to those obtained from simultations using i) a value for ΔEOH obtained from the linear relation between
ΔEO and ΔEOH (see text for more details) and ii) a ΔEOH value calculated directly by DFT in [1].
Fig. S2 Kinetic current densities for ORR on Pt(111): solid black line: measured at 10 mV s-1
in 0.1 M HClO4, dashed red line: simulated employing values for ΔEOH computed in
[1], blue dotted line: simulated employing the linear relation between ΔEO and ΔEOH in [1] (blue dotted line).
Employing the DFT calculated value of ΔEOH = 1.05 eV, the simulated kinetic current density is very close to the experimentally measured one, in good aggreement with [2;4] Minor differences might originate from the transfer coefficient arbitrarily fixed to 1. When the value of ΔEOH employed for the simulation is obtained from the binding energy of oxygen via the linear relation described in the text, this leads to lower value of ΔEOH and hence a larger offset (see Table 3 in the main paper). The discrepancy is evident also from the significant deviation between the ΔEOH value and the linear relation in figure 4 in ref. [3] for Pt metal. However, since there are no direct calculations of the ΔEOH (and ΔEOOH) values for the various Pt/Ru bimetallic surfaces employed in this study, comparitive simulations of the kinetic current densities require the use of the linear relations between ΔEO and ΔEOH and ΔEO and ΔEOOH.
References
1. Nørskov,JK, Rossmeisl,J, Logadottir,A, Lindqvist,L, Kitchin,JR, Bligaard,T, and Jónsson,H (2004) J.Phys.Chem.B 108: 17886
2. Rossmeisl,J, Karlberg,G, Jaramillo,T, and Nørskov,JK (2008) Faraday Discuss. 140: 337
3. Rossmeisl,J, Logadottir,A, and Nørskov,JK (2005) Chem.Phys. 319: 178
4. Stamenkovic,VR, Fowler,B, Mun,BS, Wang,G, Ross,PN, Lucas,CA, and Markovic,NM (2007) Science 315: 493