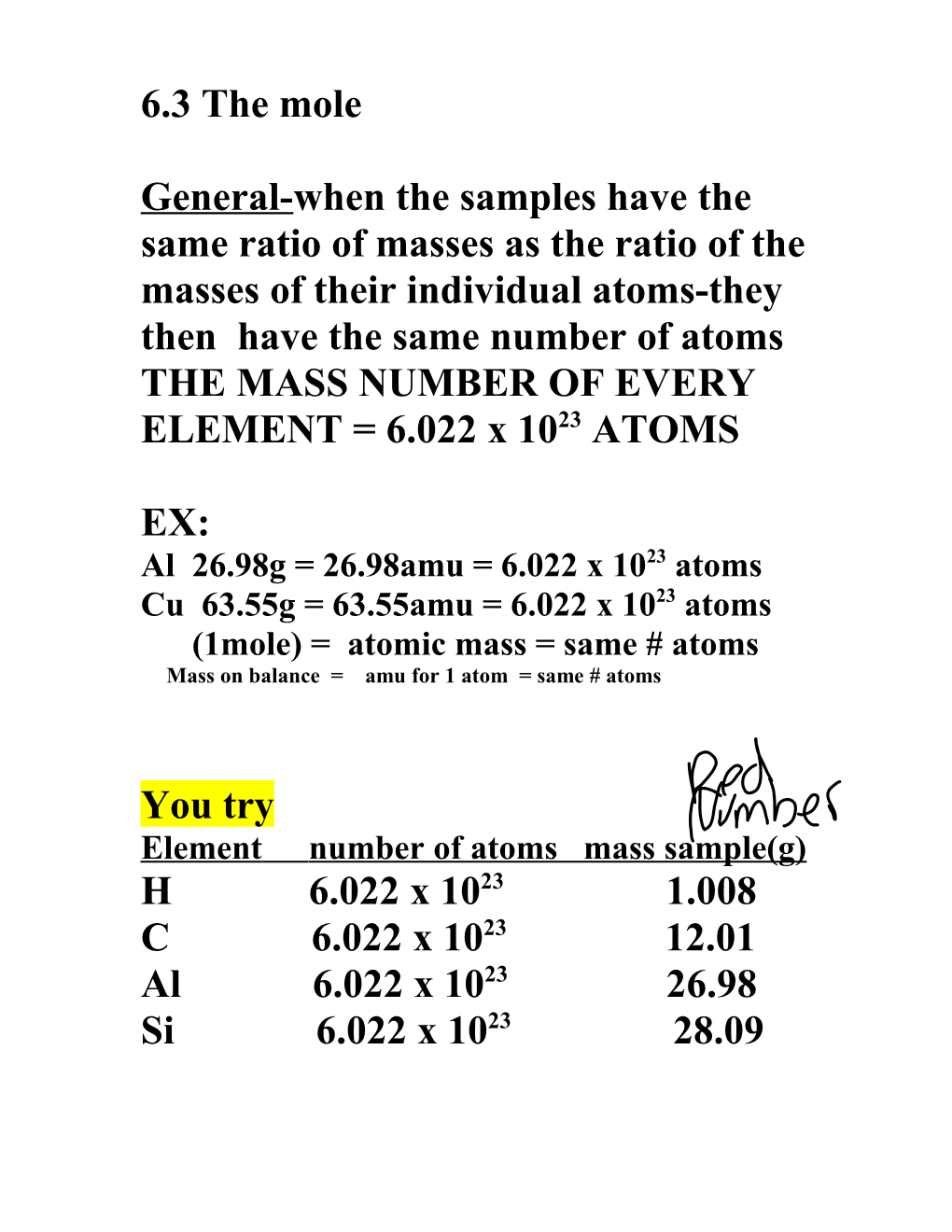
6.3 The mole
General-when the samples have the same ratio of masses as the ratio of the masses of their individual atoms-they then have the same number of atoms THE MASS NUMBER OF EVERY ELEMENT = 6.022 x 1023 ATOMS
EX: Al 26.98g = 26.98amu = 6.022 x 1023 atoms Cu 63.55g = 63.55amu = 6.022 x 1023 atoms (1mole) = atomic mass = same # atoms Mass on balance = amu for 1 atom = same # atoms
You try Element number of atoms mass sample(g) H 6.022 x 1023 1.008 C 6.022 x 1023 12.01 Al 6.022 x 1023 26.98 Si 6.022 x 1023 28.09 Mole Molar mass(red number on the big chart) it is equal to 6.022 x 1023 atoms Avogadro’s number-one mole of something equals 6.022 x 1023 atoms !! one mole of marbles is enough marbles to cover the surface of the earth 50mile depth!! -sample of an element with a mass is = to that element’s average atomic mass and is expressed in grams and contains 1 mole of atoms or 6.022 x 1023 atoms Example problems
Calculate the number of moles of and the number of atoms in a 10g sample of Al: 10gAl x 1 mol Al = .3707mol Al 26.98g Al
.3707mol Al x 6.022 x 10 23 atoms = 2.23 x 1023 atoms 1 mol Al Calculate the number of atoms in a 10g sample of Al using a string conversion:
10gAl x 1 mol Al x 6.022 x 10 23 atoms = 2.23 x 1023 atoms 26.98g Al 1 mol Al