1 UNIT 4 CHEMICAL EQUILIBRIUM
Day 1
A. Recognizing Equilibrium (pg 424)
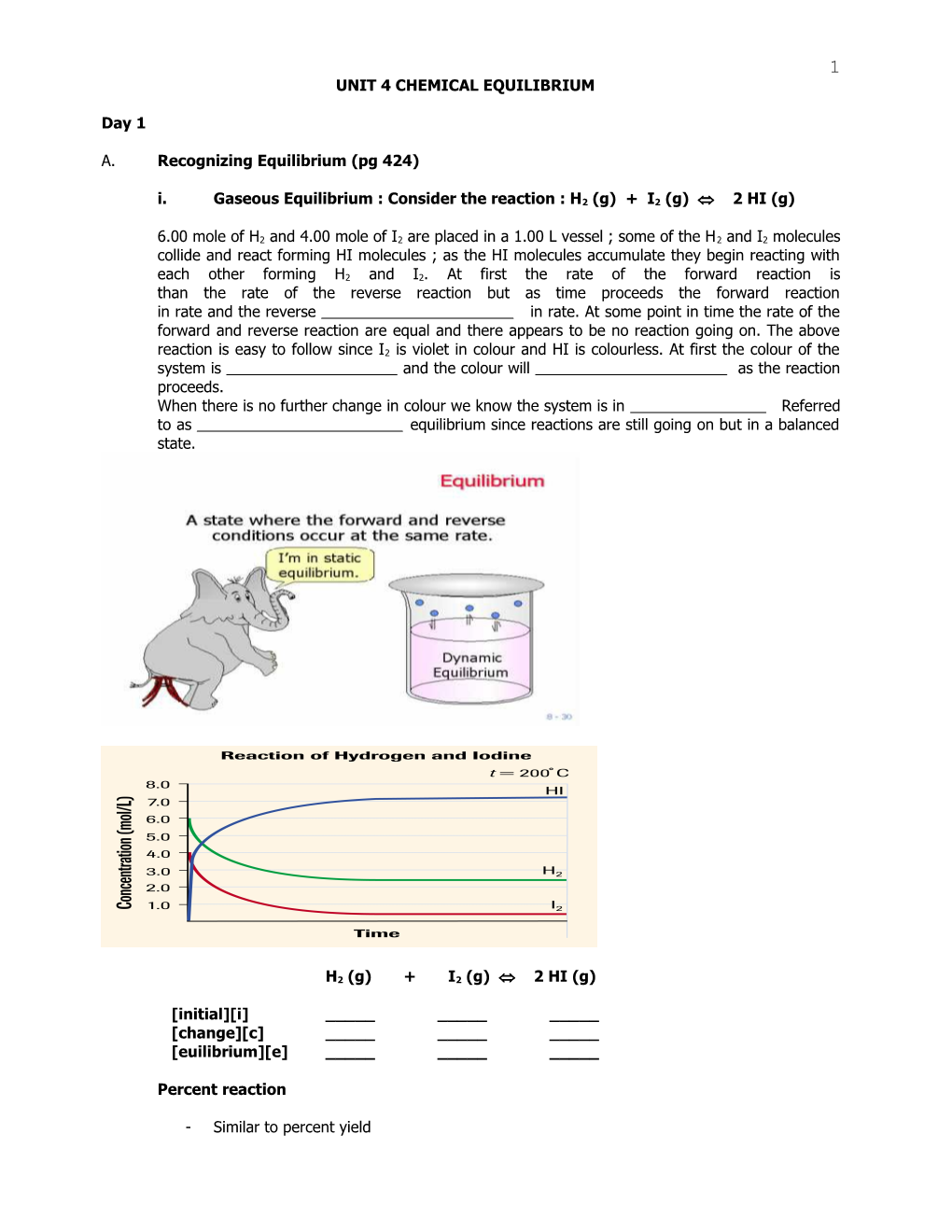
i. Gaseous Equilibrium : Consider the reaction : H2 (g) + I2 (g) 2 HI (g)
6.00 mole of H2 and 4.00 mole of I2 are placed in a 1.00 L vessel ; some of the H2 and I2 molecules collide and react forming HI molecules ; as the HI molecules accumulate they begin reacting with
each other forming H2 and I2. At first the rate of the forward reaction is than the rate of the reverse reaction but as time proceeds the forward reaction in rate and the reverse in rate. At some point in time the rate of the forward and reverse reaction are equal and there appears to be no reaction going on. The above
reaction is easy to follow since I2 is violet in colour and HI is colourless. At first the colour of the system is and the colour will as the reaction proceeds. When there is no further change in colour we know the system is in Referred to as equilibrium since reactions are still going on but in a balanced state.
H2 (g) + I2 (g) 2 HI (g)
[initial][i] ______[change][c] ______[euilibrium][e] ______
Percent reaction
- Similar to percent yield 2 - The yield of product measured at equilibrium compared with the maximum possible yield of product
Percent Reaction = Concentration at Equilibrium x 100% Theoretical yield
% Reaction =
Consider the graphs above and answer the following :
1. The initial concentration of H2 ______I2 .
2. As the reaction proceeded the concentration of H2 decreased to 2.5 mol/L and I2 decreased to 0.50 mol/L : [HI] increased from 0 to 7.0 mol/L . Why did the [HI] increased twice the value that both hydrogen and Iodine decreased by ? ______
Equilibrium Conditions
1. No input or output (______system ) 2. Presence of both species ( reactants and products ) or phases in a two phase system 3. No apparent change in macroscopic properties ( pressure, temp., colour etc.)
Consider the flasks below :
H2 (g) + I2 (g) 2 HI (g)
[initial][i] ______[change][c] ______[euilibrium][e] ______
% Reaction =
ii. Solubility Equilibrium
- consider sodium chloride dissolving in water ; the crystals begin to ______as more and more ions accumulate they start ______. When these two rates are equal the solution is said to be ______and equilibrium has been established. 3
iii. Phase equilibrium H2O (s) H2O(l)
Consider the following :
When the rate of evaporation is equal to the rate of condensation, no net change is occurring. The system is in a state of dynamic equilibrium since both events occur simultaneously. 4
1. System closed or open ______2 Presence of both species or just one ______3. Constant properties or properties changing ______4. How would you know if this system is at equilibrium or not? ______5. How does the rate of evaporation compare to the rate of condensation? ______6. How would the rates change if it was heated ?______Complete pg 428 (1-3)
N2O4 - NO2 Equilibrium(pg 430)
- consider a container is filled with colourless N2O4 (g)
Consider the equation : N2O4 (g) + energy 2NO2 (g)(reddish-brown)
- N2O4 is just 2 NO2 molecules stuck together
- the N2O4 molecules begin to break down forming ______and the colour ______; at some point the colour remains ______and ______is established.
Consider the following experimental data
Time(s) 0 20 40 60 80 100
[N2O4] 0.10 0.07 0.05 0.04 0.04 0.04
[NO2] 0.0 0.06 0.10 0.12 0.12 0.12
Questions :
1. At what time was equilibrium established ? ______2. At equilibrium which one has a higher concentration and what colour would exist ?______
3. At 20 s is the rate of the forward reaction increasing or decreasing ? ; what about the reverse reaction ______
4. If the conc. of N2O4 decreases by 0.02 ; by what value does the NO2 increase by and Why ? ______
5. Does this data reflect the graph above ? ______5
Example 2
1. The reversible reaction is : Cl2(g) + PCl3(g) PCl5(g) Consider the table below :
Reaction Time (s) [ Cl2 ] [ PCl3 ] [ PCl5 ]
0 1.00 1.00 0.00
20 0.90 0.90 0.10
40 0.80 0.80 0.20
60 0.75 0.75 0.25
80 0.71 0.71 0.29
100 0.71 0.71 0.29
120 0.71 0.71 0.29 a. When was equilibrium attained ? ______b. What would be the conc. of Cl2 after 20 hours ? ______c. After 40 s which reaction is faster ? ( forward or reverse ) ______d. At 25 s is the forward reaction increasing or decreasing in its rate ? ______e. At 25 s is the reverse reaction increasing or decreasing in its rate ? ______f. At 100 s which reaction is faster, forward or reverse? ______g. At 100 s which reaction is increasing in its rate, the forward or the reverse? ______
Ex.3 Consider the equilibrium : N2 + 3 H2 2 NH3
1.0 mol of N2 and 1.0 mol of H2 are placed in a 1.0 L flask and allowed to react. Consider the table below :
Reaction Time [ N2 ] [ H2 ] [ NH3 ]
0.0 min. 1.00 1.00 0.00
1.0 0.90 0.70 0.20
2.0 0.80 0.40 0.40
3.0 0.75 0.25 0.50
4.0 0.72 0.16 0.56
5.0 0.70 ______
6.0 ______0.60 a. Fill in the blanks in the table. b. When was equilibrium established ? ______c. What is equal at equilibrium ? ______d. Account for the change in concentration for both reactants and products. ______e. If all the N2 had been converted to NH3 . How many moles of ammonia would form ? ______f. Calculate the % yield considering the amount of ammonia that is found at equilibrium ? ______6 Complete pg 437( 1,3 )
Day 2 : Equilibrium Calculations (pg 431)
- consider the equilibrium N2O4 2 NO2
1.00 mole of N2O4 is placed in a 2.0 L container and allowed to come to equilibrium. At equilibrium
there is 0.200 mole of N2O4 calculate the concentration of NO2
N2O4 2 NO2 [initial][i] ______[change][c] ______[euilibrium][e] ______
To calculate the theoretical yield or percent reaction ; assume all the N2O4 is converted to NO2. The theoretical yield would be ______What is the % yield ? ______
Complete pg 437 ( 6,7,4 ) pg 438 (7a-c,8,9)
Law Of Chemical Equilibrium (pg 439) 7
- consider the general equation : aA + bB cC + dD
Ke = kf/kr = [C]c[D]d ------= Equilibrium constant [A]a[B]b
[ ] - refers to ______in the units of ______
- Consider the reaction below :
2H2(g) + O2(g) 2H2O (g)
2 Equilibrium constant Ke = [H2O] ------Mass action expression 2 [H2] [O2]
- Ke is determined by dividing product of the conc. of products by the product of the conc. of reactants - high Ke values indicate that the ______are favored over the ______Write the Ke expressions for the following and state which is favored at equ. reactants of products ; also note that solids and liquids do not appear in equ. expressions only gases and aqueous solutions.
-2 1. 2 HI (g) I2(g) + H2(g) Ke = 1.84 x 10 = at 423oC
2. AgCl(s) Ag+(aq) + Cl-(aq) Ke = 1.7 x 10-10 = at 25oC
3. SO2(g) + 1/2 O2(g) SO3(g) Ke = 7.7 =
2 4. 2 C(s) + O2(g) 2 CO(g) Ke = 1.7 x 10 =
General Rule : If Ke is > 1 than conc. of products is ______conc of reactants If Ke is < 1 than conc. of products is ______conc. of reactants 8
Magnitude of Ke : The higher the Ke the greater extent the reaction has gone in the forward direction that is toward completion
Consider the equ. Cu(s) + 2 Ag+(aq) Cu2+(aq) + 2 Ag(s) Ke = 2.0 x 1015
Write the Ke expression : Ke =
Because of the high value the are favored over the ______and thus the ______reaction is favoured over the ______reaction.
- If the Ke for the following equ. is 50 : H2 + I2 2 HI than what would be Ke for the following :
a. 1/2 H2 + 1/2 I2 HI Ke =
b. 2 HI I2 + H2 Ke =
Complete pg 442 (1) pg 444 (4-6) pg 447 (7) 9
Calculating Ke values(pg 443)
1. Given the equ. : 2 A(g) + B(g) C(g) + 2 D(g)
The following equ. conc. were found [A] = 2.0 moL/L ; [B] = 3.0 mol/L ; [C]= 4.0 mol/L ; [D] = 1.0 mol/L
Calculate Ke = = 0.33
2. Given the equ. : A(g) + 2B(g) 2C(g) 1.0 mole of A and 2.0 moles of B are placed in a 1.0 L vessel. At equ. there was 0.50 moles of A. Calculate the equ. conc. of B and C and calculate the Ke value.
A(g) + 2 B(g) 2 C(g) [initial] [i] ______
[change in] [c] ______
[final] [e] ______
Ke = =
3. Consider the equ. : H2 + I2 2 HI
0.10 moles of H2 and 0.20 moles of I2 are placed in a 2.0 L container. When equ. was established it
was found that 20 % of the H2 had reacted. Calculate the equ. conc. of each and calculate the Ke value.
H2 + I2 2 HI
[initial] [i] ______
[change in] [c] ______
[equ.] [e] ______
Ke = =
4. Consider the equ . 2 SO2(g) + O2(g) 2 SO3
A 10.0 L vessel was filled with 4.0 mol SO2 , 2.2 mol O2 and 5.6 mol of SO3. When equ. is
established there are 2.6 mol of SO2. Calculate equ. conc of each and calculate the Ke value.
2 SO2 + O2 2 SO3
[initial] [i] ______
[change in][c] ______
[final] [e] ______10
Ke = = = 48
Complete pg 449 ( 3,4,6,8,9 )
Day 3 Factors affecting the State of Equlibrium
Le Châteliers Principle(pg 450)
- if a system at equilibrium is subjected to change or ______processes tend to occur to counteract the stress
1. Concentration (pg 450)
- consider the equilibrium : Fe3+ ( aq ) + SCN- FeSCN2+ ( yellow ) ( colourless ) ( deep red )
- adding more SCN- ----> - stress too much SCN- to relieve stress equilibrium shifts to the ______producing more causing the colour to ______. - adding more Fe3+ has equal affect; See graph below 11
Ex. 2 N2 ( g ) + 3 H2 (g) 2 NH3 ( g )
- increase conc. of N2 -----> stress too much to relieve stress
equilibrium shifts to the ; thus conc. of H2 will and
conc. of NH3 will ______Consider the equ. conc to be N2 = 1.0 moL/L; H2 = 3.0 moL/L
and NH3 = 2.0 moL/L
If an additional 0.5 moL/L of N2 is added; graphically show how the equ. conc are established; keep in mind the mole ratios from the equation.
Graph :
H2
NH3
N2
Ex. 3 Consider the equ. SO2(g) + 1/2 O2 (g) SO3(g)
What would be the effect on the equilibrium conc. of all substances in the following instances . Illustrate on the graphs that follow:
a. More O2 is pumped in :
effect on [ SO2 ]
effect on [ O2 ]
effect on [ SO3 ]
b. Some SO2 is removed from the system :
effect on [ SO2 ]
effect on [ O2 ]
effect on [ SO3 ] 12
[SO3]
[SO2]
[O2]
[SO3]
[SO 2]
[O 2]
- it is important to understand that only substances in the gaseous state or in aqueous form can affect equilibrium ; that is the addition of solids will not affect the equilibrium position.
Consider the equ. : FeO (s) + CO(g) Fe (s) + CO2 (g)
What would be the effect on the equ. conc. of CO2 as a result of the following ?Answer each one as follows the stress is ; to relieve the stress equ.shifts to the
and the equ. conc. of CO2 will ______
Stress Equilibrium Shift [CO2 ] Increase [CO] Add more FeO
Decrease [CO2 ] i. ______ii. ______iii. ______
Review : 2A(g) + B (g) 4C(g) + 3D(g) A(g) + B (g) C(g) + D(g) Some A is added Some A is added 2x 4x [A] [C] [A] [C]
time time time time New Equilibrium is established New Equilibrium is established
3x [B] [D] [B] [D] x
time time time time
2. Effect of Temperature (pg 453)
- consider the equ. N2O4 (g) + 59 kJ 2 NO2 (g) - increase in temp. ; stress is too much energy to relieve the stress equ. shifts to the 13 using up some of the energy ; reddish brown colour ______since there increase in conc. and a decrease in conc.
[N2O4]
[NO2]
Ex.2 consider equ. N2 (g) + 3 H2 (g) 2 NH3 (g) + energy
- increase in temp. ; stress is to relieve stress equ. shifts to the
thus conc. of NH3 - decrease in temp. ; stress is to relieve stress equ. shifts to the
; [ NH3] ______
H2
NH3
N2
Ex.3 Consider equ. N2 (g) + 1/2 O2 (g) + energy N2O (g) Complete graph below if the temp. is suddenly increased.
[N2]
[O2]
[N2O]
Ex. 4 Consider equ. C2H4 (g) + H2 (g) C2H6 (g) + energy Complete the graph below if there was a sudden decrease in temp.
[C2H4]
[H2]
[C2H6]
3. Effect of Pressure (pg 454)
N2O4 (g) + energy 2 NO2 (g)
- consider a sudden decrease in volume ; partial pressure of gases would colour of gases would ; lasts for a long moment then colour ______and becomes constant.
- stress is too much pressure ; to relieve stress equ. shifts to the since mole(s) exerts less pressure than mole(s);
# of moles of NO2 will and # of moles of N2O4 will but conc. of 14 both will ______
Diagram :
[N2O4]
[NO2]
Ex.2 Consider the equ. N2(g) + 3 H2(g) 2 NH3(g) - Consider a sudden increase in volume : partial pressure of gases ______; stress is not enough pressure equ. shifts to the since moles exerts more pressure than moles ;
# of moles of NH3 and # moles of N2 and H2 both But the conc. of each ______
Diagram :
H2
NH3
N2
Pressure affects equilibrium only if :
1. gases are involved not solids or liquids 2. # of moles of gas on the left and right of the equation are different.
Ex.2 Consider the equilibrium : SO2(g) + 1/2 O2(g) SO3(g)
If the volume was decreased ; what effect would have the # of moles of each substance when equilibrium is re-established ? Also indicate the effect on the conc. of each.
Substance # of moles Concentration
SO2
O2
SO3
[SO3]
[SO2]
[O2]
- Consider the Equ.: CO2 (g) CO2 (aq) + energy 15 One brand of soft drink is bottled under a pressure of 101.3 KPa. A competing brand is bottled
under 202.6 KPa. How does this affect the conc. of CO2 in the drink ? Stress is
to relieve stress equ. shifts to the thus [CO2(aq)]
In the bottles of soft drink at constant temp. CO2(aq) is in equ. the CO2(g) present in the space above the liquid. If P is constant ;
a) How does the [CO2(aq)] change when the drink is refrigerated ? Stress is to relieve stress equ. shifts to the
______[CO2(aq)] ______b) How does [ CO2 (aq) ] change if the drink is warmed ?
Stress is to relieve stress equ. shifts to the [ CO2(aq) ] ______
Review : 2A(g)+B (g) + heat 4C(g) + 3D(g)
The temperature is increased 4x [A] [C] 2x
time time New Equilibrium is established
3x [B] x [D]
time time
IV Effect of a Catalyst (pg 455)
- a catalyst speeds up a reaction since less energy is needed but both the forward and reverse or affected by the same amount so that equilibrium is established ______but it has no overall effect on the equilibrium. V Adding Inert Gases
- adding a noble gas such as He does not affect equilibrium as long as the volume remain constant ; if the pressure remains constant the volume must increase thus is a decrease in partial pressures of gases ; equilibrium shifts to the side containing more moles.
Day 4 Consider the Equ. 2 H2O(g) + heat 2 H2(g) + O2(g)
1. increase [H2] - stress is to relieve stress equ. shifts to the
; conc. H2 , [O2] [H2O] ______
2. decrease [H2O] -stress is to relieve stress equ. shifts to the
; [H2] ; [O2] ; [H2O] ______
3. increase [O2] - stress is ; to relieve stress equ. shifts to the [H2]
; [O2] ; [H2O] ______4. increase temp.- stress is ; to relieve stress equ. shifts to the
______[H2] ; [O2] ; [H2O] ______16
5. decrease volume - stress is ; to relieve stress equ. shifts to the
since there are moles of gas on that side of the equation; # moles H2
, # of moles O2 , # of moles of H2O _____ ;
[H2] , [O2] ,[H2O] .
6. add an inert gas ( helium) so that the volume is constant. - with the addition of helium the total pressure will ; the partial pressure of the
gases H2, O2 and H2O will ______; thus stress on the system is ______and equ.______
7. add an inert gas so that the pressure remains constant - for pressure to remain constant the volume must ______; this will ______the partial pressure of the reactants and products ; stress is ______to relieve stress equ. shifts to the ______since there are ______moles on that side.
# of moles H2 ______; O2 ______and # of moles of H2O ______; [H2] ______
[O2] ______[H2O] ______
8. A catalyst is added ______
Complete pg 457 ( 1-6) pg 459 (2,3,5,6,8,12)
Dynamic Equilibrium 17 When the _ _ _ _ _ in a chemical reaction looks like this ⇌ , it shows that the reaction is missing words ______. This means the products can react together and turn back into the added ______reactants. In other words, the reaction can go _ _ _ _ ways. any When a reversible reaction is set up in a ______container, the forward reaction happens arrow much faster than the reverse reaction at first because there's a high ______both of reactants and hardly catalysts _ _ _ products. As _ _ _ _ goes on, however, the concentration of reactants falls and the closed concentration of products rises. The forward reaction gets gradually slower and the concentration counteract ______reaction gets gradually faster. It follows that there must come a time when the directions reactions are happening in both ______at the same _ _ _ _ and the ______dynamic _ _ _ of reactants and products stop changing. This balanced situation is called ______endothermic equilibrium. It can only be achieved in a ______system where no materials can _ _ _ _ _ equally _ or be _ _ _ _ _. equilibrium A ______called Le Chatelier ______what happens when the escape conditions of reactions in ______are changed. He developed this ______exothermic _ _: 'If a reaction in equilibrium is subjected to a change, then it responds in such a way as extra to ______the effect of that change'. In other words; if you change it, it tries to gaseous change back! investigated Imagine a reversible reaction where the forward reaction is ______and the lowers reverse reaction is endothermic. If we raise the ______, Le Chatelier's principle original tells us that more ______reverse reaction will happen to remove the _ _ _ _ _ position heat we added. The quantity of reactants will increase ______to the quantity of pressure products and we say that the equilibrium position has shifted in favour of the reactants. principle Now imagine a reversible reaction where the ______of gaseous reactants is greater than quantities the volume of ______products. In this situation the forward reaction decreases gas raise volume and, therefore, ______the ______. If we _ _ _ _ _ the pressure, Le rate reaction Chatelier's principle tells us that more forward ______will happen to remove the extra relative pressure we added. The equilibrium ______will _ _ _ _ _ in favour of the products. reverse Le Chatelier found that adding ______to reversible reactions in closed systems does reversible not change the quantities of reactants and products at equilibrium. He observed, however, scientist that adding catalysts ______up the forward and reverse reactions ______so that sealed dynamic equilibrium is reached more quickly. shift speeds ⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌⇌ temperature time volume
According to Le Chatelier's principle, which conditions ΔH for the temperature pressure catalyst t r r r t n
give the best yield of products for these chemical forward e e e n h h e h h h e w w g g s t t t i i s i i i o reactions. (Tick neither if changing the condition reaction o e l l h h b e e e -1 r a n n n makes no difference to the equilibrium position.) (kJmol ) p
1. N2 (g) + 3H2 (g) ⇌ 2NH3 (g) -92
2. N2O4 (g) ⇌ 2NO2 (g) +58
3. H2 (g) + CO2 (g) ⇌ CO (g) + H2O (g) +40
4. 4NH3 (g) + 5O2 (g) ⇌ 4NO (g) + 6H2O (g) -909
5. 2SO3 (g) ⇌ 2SO2 (g) + O2 (g) +196
6. 2NO (g) + O2 (g) ⇌ 2NO2 (g) -115
7. 3N2O4 (g) + 2H2O (l) ⇌ 4HNO3 (aq) + 2NO (g) -103
8. 3NO2 (g) + H2O (l) ⇌ 2HNO3 (aq) + NO (g) -117
9. CO (g) + 2H2 (g) ⇌ CH3OH (g) -92
10. PCl5 (g) ⇌ PCl3 (g) + Cl2 (g) +124
Reading Assignment : Haber Process (pg 461) Complete pg 462 ( 3-5 ) 18
Complete pg 462 ( 3-5 )
Case Study: The Haber Process: Ammonia for Food and Bombs
- N2 (g) + 3 H2 (g) 2 NH3 (g) + heat
- to increase amount of product made, Le Chatelier’s principle indicates:
- increase [ reactants] – continuous addition of N2 and H2 o - decrease [products] – cool bottom of reaction container – ammonia condenses at – 33 C, cooled nitrogen and hydrogen gases are recycled back to the top of the reaction container so are not wasted
- increase pressure – happens at about 50 Mpa ( 50 atmospheres ) pressure
- decrease temp – problem – at low temp, rate forward reaction so slow that it is uneconomical o - so temp kept high , at 500 C to speed up reaction and the other 3 factors compensate
- use a catalyst to speed up reaction further – discovery of an effective catalyst got Haber the Nobel prize 19 footnote: Haber was a good German citizen and helped invent mustard gas for Germany to use as a weapon during WW1 – one of the first uses of chemical weapons and devastating to any who were exposed to it. - many people objected to him being awarded the Nobel prize because of this other part of his work
The Haber Process & Optimum Conditions ⇌⇌⇌ The Haber Process is an important ______process because it's used to missing words
make the gas ______(NH3). Ammonia is a _ _ _ material for the manufacture of ______and these are vital when it comes to growing food for the added iron world's enlarging ______. Ammonium nitrate is a particularly important ammonia liquid fertilizer. It's made from ammonia using this ______reaction: arrow lots atmospheres low ammonia + nitric acid ammonium nitrate ⇌ base lower NH3 (aq) + HNO3 (aq) ⇌ NH4NO3 (aq) better mixture During the Haber Process, ______gas from the air and hydrogen _ _ _ boiling moderate from water or ______gas are fed into a reaction ______. Here they catalysts natural combine to form ammonia: chamber neutralization -1 N2 (g) + 3H2 (g) ⇌ 2NH3 (g) (ΔH = -92 kjmol ) chemicals nitrogen We can see from the _ _ _ _ _ that the Haber Process reaction is chosen pipes ______. As such the reaction will reach equilibrium after a time and the compromise population reaction chamber will contain a ______mixture of nitrogen, hydrogen and condenser position ammonia. This ______is piped from the reaction chamber into a conditions pressure ______where the ______is lowered to a level just below consider principle the boiling point of ammonia. The ammonia gas then condenses into ______equilibrium priorities ammonia so it can be ______off at the condenser _ _ _ _. Since the _ _ _ _ _ even quickly _ _ points of nitrogen and ______are _ _ _ _ _ than ammonia, these two _ exothermic rate ______remain as gases and can be ______. They pass through _ _ expensive raw _ _ _ back into the reaction chamber. factors reasonable The ______in the Haber Process reaction chamber have been carefully faster recycled ______by industrial ______who must consider two main fertilizers reverse ______; they want conditions that produce _ _ _ _ of ammonia as forward reversible ______as possible. In other words, they must ______both _ _ _ _ _ four running and reaction _ _ _ _. gas scientists gaseous slow hazardous speed high tapped hydrogen temperature improve two industrial yield Since the forward reaction of the Haber Process is ______, Le Chatelier's ______tells us that a _ _ _ temperature will shift the equilibrium ______in favour of the products to give a good yield of ammonia. Unfortunately, a low temperature also makes the reaction very _ _ _ _ and so scientists have had to ______. They have opted for a ______temperature (450oC) which gives a reasonable yield at a ______rate. The ______reaction in the Haber Process lowers the ______because _ _ _ _ volumes of nitrogen and hydrogen react to produce only _ _ _ volumes of ammonia. In this situation Le Chatelier's principle tells us that a _ _ _ _ pressure is best for producing a high yield of ammonia and high pressure conditions have the _ _ _ _ _ benefit of making chemical reactions happen ______. Scientists have not had to compromise with pressure and have opted for a high pressure of 200 ______. An even higher pressure might seem like a good idea because it would produce a ______yield _ _ _ _ more quickly, however, there are other ______to consider. Setting up and ______high pressure factories is ______and potentially ______. An _ _ _ _ catalyst is used in the Haber Process. ______do not affect the position of equilibrium for reversible reactions; they simply make both forward and ______reactions happen faster so that ______is reached more quickly. Scientists have opted to use iron for _ _ _ _ _; it does not ______the yield of ammonia at all. 20
Day 5 : Lab pg 514 “Le Chatelier’s Principle
Independent Research :
Analyse the optimal conditions for a specific chemical process related to the principles of equilibrium that takes place in nature or is used in industry (e.g., the production of sulphuric acid, electrolyte balance in the human body,sedimentation in water systems) Sample issue: The principle of dynamic equilibrium is used in industrial processes to maximize the concentration of products and minimize leftover reactants. Industrial chemists determine ideal pressure and temperature conditions, and proper catalysts, so that fewer materials and less energy are used. Questions:
Why are low temperature conditions not used with exothermic reactions?
______
How do chemicals dissolved in human blood helpmaintain a blood pH level between 7.2 and 7.4?
______21
Review Exercises In the problems below, for each change given in the first column of the table, use Le Chatelier's principle to predict • the direction of shift of the equilibrium. • the effect on the quantity in the third column. 1. For the following reaction o 5 CO(g) + I2O5(s) I2(g) + 5 CO2(g) H = -1175 kJ for each change listed, predict the equilibrium shift and the effect on the indicated quantity.
Direction Effect on Effect Change of Shift Quantity (increase, decrease, ( ; ; or no or no change) change) (a) decrease in volume Ke (b) raise temperature amount of CO(g) (c) addition of I2O5(s) amount of CO(g) (d) addition of CO2(g) amount of I2O5(s) (e) removal of I2(g) amount of CO2(g)
2. Consider the following equilibrium system in a closed container:
o Ni(s) + 4 CO(g) Ni(CO)4(g) H = - 161 kJ
In which direction will the equilibrium shift in response to each change, and what will be the effect on the indicated quantity? Direction Effect on Effect Change of Shift Quantity (increase, decrease, ( ; ; or no or no change) change) (a) add Ni(s) Ni(CO)4(g) (b) raise temperature Ke (c) add CO(g) amount of Ni(s) (d) remove Ni(CO)4(g) CO(g) (e) decrease in volume Ni(CO)4(g) (f) lower temperature CO(g) (g) remove CO(g) Ke
Le Chatelier's Principle Exercises
For each of the following indicate the direction the equilibrium would shift and what would happen to the concentrations of each substance in the equilibrium 22
1. The following equilibrium reaction may be established with carbon dioxide and steam
è CO (g) + H2O (g) < CO2 (g) + H2 (g) + heat
What would be the effect of each of the following on the equilibrium:
(a) The addition of more H20?
(b) The removal of some H2?
(c) Raising the temperature ?
(d) Increasing the pressure?
(e) Addition of a catalyst?
2. What would be the effect on each of the following on the equilibrium involving the synthesis of methanol? è CO (g) + 2 H2 (g) < CH3OH (g)
(a) The removal of CH3OH .
(b) an increase in pressure
(c) lowering the concentration of H2.
(d) the addition of a catalyst
3. A small percentage of nitrogen gas and oxygen gas in the air combine at the high temperatures found in automobile engines to produce NO (g) which is air pollutant.
è N2 (g) + O2 (g) + Heat < 2 NO (g)
(a) Higher engine temperatures are used to minimize carbon monoxide production. What effect do these higher engine temperatures have on the production of NO (g)? Why?
(b) What effect would high pressures have on the production of NO? Why?
4. What would be the effect of each of the following on the equilibrium involving the reaction of coke, C
(s), with steam to give CO and H2?
è C (s) + H2O (g) < CO (g) + H2 (g) 23
(a) The addition of steam?
(b) An increase in pressure?
(c) The removal of H2 as it is produced?
(d) The addition of a catalyst?
5. The binding of oxygen by hemoglobin (abbreviated Hb), giving oxyhemoglobin (HbO2) is partially + regulated by the concentration of H and CO2 in the blood. Although the equilibrium is rather complicated it can be summarized as + è + HbO2 + H + CO2 < CO2HbH + O2 .
According to Le Chatelier's principle what would be the effect of each of the following on the equilibrium
+ (a) The production of lactic acid (contains H ) and CO2 in a muscle during exercise.
(b) Inhaling fresh oxygen enriched air
Day 6 :Equilibrium Problems (pg 463) :
A. Reaction Quotient (Q) Which way will the equilibrium shift ? If Q is < Ke equ. will shift to the ______; If Q > Ke equ. will shift to the ______
Consider the equ. N2(g) + 3 H2(g) 2 NH3(g) Ke = 0.064 . The concentrations are as follows
[ N2 ] = 0.20 mol/L [H2] = 0.085 [NH3] = 0.80 mol/L
i. Calculate trial Ke or Q =
ii. Compare Q to Ke ______; equilibrium should shift to the ______
Try pg 465 (1,2)
B. Given Ke determine equ. conc. 24 1. Consider the equ. 2 A(s) + 3 B(g) 2 C (g) + D (s) Ke = 1.0 x 10-2. If at equ. the conc. of B was found to be 0.20 mol/L; What is the equ. conc. of C? Let equilibrium concentration of c = x
2 A(s) + 3 B(g) 2 C (g) + D (s) [equ.] ______
Ke =
[C] =
Practice pg 466 (3,4) C. Given initial conc. And Ke find equ conc. ( pg 465 )
Consider the equ. A(g) + B(g) 2 C (g) Ke = 25.0 If 0.50 mol of A and 0.50 mol of B are placed in a 5.0 L container; calculate the equ. conc. of each substance.
A(g) + B(g) 2 C (g) [I] ______
[C] ______
[E] ______
Since there is no C equ. shifts to the right . Let x = [A]reacting
To simplify take the square root of each side
Ke =
x =
[A] = [B] = [C] =
3. Consider the equ. A(g) + B(g) 2 C (g) Ke = 27
3.0 moles of A, B and C are placed in a 2.00 L container. Calculate the equ conc. of each.
A(g) + B(g) 2 C (g)
[I] ______
[C] ______
[E] ______
i. Calculate Q to see which way the equ. Will towards
ii. Let x =[change in concentration of substance reacting]. Fill in chart above. iii. Ke = 25
iv. x = [A] = ____ [B] = _____ [C] = ______
Complete pg 472 (5,6)
d. Given initial conc. and Ke find equ conc. ( imperfect square ; simplifying assumption required.
-6 Consider the equilibrium : 2H2S(g) 2H2(g) + S2(g) Ke = 4.20x10
0.200 mol of H2S is placed in a 1.00 L vessel. When equilibrium is established what is the concentration of each ?
2H2S(g) 2H2(g) + S2(g)
[i] ______[c] ______[e] ______
Let change in concentration of H2S be ______. Fill in the rest of the table,
If Ke is very small assume 0.200 – 2x is 0.20 . No quadratic is needed. You must do the following check 2x/.200 x 100% must be less than 5%
Ke =
X = ______. Do check. [H2S] ______[H2 ] = ______[S2]
Practice pg 476 # 8
Day 7 e. Same as above but Quadratic is needed (pg 476)
-3 1. Given the equ. N2O4(g) 2 NO2(g) Ke = 6.7 x 10
0.060 mole of NO2 and 0.85 mole of N2O4 are placed in a 1.00 L vessel. When equ. Is established;
What are the equ. conc. of NO2 and N2O4?
Step 1 Calculate a trial Ke(Q) to see which way the equ. will shift 26
Step 2 N2O4 2 NO2 Let x =
[initial] ______Quadratic is needed [change] ______[final] ______
Ke =
X= ______[N2O4] = ______[NO2] = ______
Complete page 480 (10) and pg 481(1,2,3,6,8) 27 Equilibrium Review
1. A 1.0 L reaction vessel contained 0.750 mol of CO (g) and 0.275 mol of H2O (g). After one hour,
equilibrium was reached according to the equation: CO (g) + H2O (g) CO2 (g) + H2 (g). Analysis
showed that 0.25 mol of CO2 was present. Calculate Kc. ( 5 )
2. Consider the equilibrium: 3 I2 (g) + 6 F2 (g) 2 IF5 (g) + I4F2 (g). At a certain temperature,
6.0 mol of IF5 and 8.0 mol of I4F2 are introduced into a 5.0 L container. At equilibrium, 6.0 mol of -4 I4F2 are left. Calculate Kc. ( 5.8 x 10 )
3. At a certain temperature, Kc = 4 for the equilibrium: 2 HF (g) H2 (g) + F2 (g). Predict the direction (if at all) in which the equilibrium will shift when the following amounts of gases are introduced into a 5.0 L vessel.
a) 3.0 mol HF, 2.0 mol H2, 4.0 mol F2 ( shift right )
b) 0.2 mol HF, 0.5 mol H2, 0.6 mol F2 ( shift left )
c) 0.3 mol HF, 1.8 mol H2, 0.2 mol F2 ( no shift )
4. For the equilibrium: Br2 (g) + Cl2 (g) 2 BrCl (g), Kc = 7.0 at 400 K. If 0.080 mol of Br2(g) and
0.060 mol of Cl2 (g) are introduced into a 2.0 L vessel at 400 K, determine the equilibrium
concentrations of all three gases. ( Br2=0.02M, Cl2=0.01M, BrCl=0.04M ) QUADRATIC EQUATION
o -2 5. At 425 C, Kc = 1.82 x 10 for the equilibrium: 2 HI (g) H2 (g) + I2 (g). If equilibrium is reached by adding only HI (g) to a 1.0 L reaction vessel, determine:
a) the concentrations of H2 (g) and I2 (g) in equilibrium with 0.0100 mol/L HI (g); -3 (H2=I2=1.35x10 ) b) the initial concentration of HI (g) placed in the vessel; ( 0.013 mol/L ) c) the percent of the original HI (g) that dissociated to reach equilibrium. ( 21%)
6. Consider the equilibrium: CO (g) + H2O (g) CO2 (g) + H2 (g). A mixture containing 1.00 mol
of CO (g) and 1.00 mol of H2O (g) is placed in a 10.00 L container at 800 K. At equilibrium, 0.665
mol of CO2 and 0.665 mol of H2 are present. Determine:
a) the equilibrium concentrations of all four gases; ( CO=H2O= 0.0335M,
CO2=H2=0.0665M )
b) the value of Kc at 800 K. ( 3.9 )
7. At 585 K, NOCl (g) is 56.4 % dissociated to reach equilibrium according to the system:
2 NOCl (g) 2 NO (g) + Cl2 (g). If 1.00 mol of NOCl (g) was placed in a 1.00 L vessel, determine:
a) the equilibrium concentrations of all three gases;
( .436mol/L,NO=0.564mol/L,Cl2=0.282mol/L)
b) the value of Kc at 585 K. ( 0.47 )
8. The reaction: A (g) + B (g) C (g) is exothermic as written. Assume you have an equilibrium mixture of A, B, and C. How would the equilibrium concentration of C (g) change with:
a) an increase in temperature; ( C decreases ) b) an increase in pressure due to a decrease in volume; ( C increases ) c) the addition of A (g); ( C increases ) d) the addition of a catalyst; ( C no effect ) e) the removal of B (g). ( C decreases ) 28 9. For each of the following equilibrium systems:
i) write the expression for Kc; ii) state the direction in which each would be shifted upon the application of the stress listed after each equation.
a) 2 SO2 (g) + O2 (g) 2 SO3 (g) + energy decrease temperature ( shift R )
b) C (s) + CO2 (g) + energy 2 CO (g) increase temperature ( shift R )
c) N2O4 (g) 2 NO2 (g) increase pressure by decreasing volume ( shift L)
d) CO (g) + H2O (g) CO2 (g) + H2(g) decrease pressure by increasing volume ( no shift)
e) 2 NOBr (g) 2 NO (g) + Br2 (g) decrease pressure by increasing volume ( shift R )
f) 3 Fe (s) + 4 H2O (g) Fe3O4 (s) + 4 H2 (g) add Fe (s) ( no shift)
g) 2 SO2 (g) + O2 (g) 2 SO3 (g) add a catalyst ( no shift)
h) CaCO3 (s) CaO (s) + CO2 (g) remove CO2 (g) ( shift R )
i) N2 (g) + 3 H2 (g) 2 NH3 (g)
add H2 (g) ( shift R )
11. Consider the following equilibria which occur simultaneously in the same solution in the presence of undissolved CA (s):
1- 1+ 1- 1. A (aq) + H (aq) B (aq) + H2O (l) 2. CA (s) A1- (aq) + C1+ (aq)
Use Le Châtelier's Principle to predict each of the following changes in:
a) the amount of undissolved CA (s) if the concentration of H1+ (aq) is increased; (decrease) b) the concentration of B1- (aq) if the concentration of C1+ (aq) is decreased; (increase) c) the concentration of H1+ (aq) if the concentration of C1+ (aq) is increased; (increase) d) the concentration of B1- (aq) if more solid CA (s) is added. ( no effect ) 29 Day 8 Solubility Product Constant (pg 482)
Ksp is defined as the product of molar concentrations of ions in a saturated solution, each raised to the appropriate power. The following table is the Solubility Product Constant for various salts at 250C:
Salt Ksp Salt Ksp Salt Ksp
-3 -21 -9 AgCH3COO 1.9 x 10 AlPO4 9.8 x 10 NiCO3 6.6 x 10
-12 -9 -8 Ag2CO3 8.4 x 10 BaCO3 2.6 x 10 PbSO4 1.8 x 10
-10 -10 -33 AgCl 1.8 x 10 BaSO4 1.1 x 10 Zn3(PO4)2 9.1 x 10
-17 -17 -5 AgI 8.5 x 10 Fe(OH)2 4.9 x 10 Ag2SO4 1.2 x 10
-33 -19 Al(OH)3 1.9 x 10 FeS 1.6 x 10
In general, the lower the Ksp value, the more insoluble the salt will be (i.e attraction between oppositely charged ions is very strong).
e.g Ag2CO3 is much more ______than Zn3(PO4)2. It is appropriate to compare Ksp values only if the charge ratio of the cations and anions are the same.
Write Ksp expressions for the following salts:
2+ - 2 1. PbCl2 Ksp = [Pb ][Cl ]
2. CaCO3 Ksp =
3. Ca3(PO4)2 Ksp =
4. Al2(CO3)3 Ksp =
5. Fe(OH)3 Ksp =
6. CuS Ksp =
7. BaSO4 Ksp =
8. Al2S3 Ksp = 30
Problems : Calculating solubility in moles/L and g/L (pg 484)
Example 1:
-9 2+ Ksp = 8.30 x 10 for PbI2. What is the molar solubility of PbI2. (i.e )[Pb ] in a saturated solution of PbI2?
2+ - PbI2(s) Pb + 2I [initial] 0 0 [] +x +2x [final] x 2x
Step 1. Let molar solubility = x = [Pb2+]
2+ - 2 Step 2. Setup Ksp expression: ksp = [Pb ][I ]
x =
Example 2:
-12 Ksp for Mg(OH)2 is 8.9 x 10 . What is the molar solubility of Mg(OH)2 in moles/L and g/L ?
2+ Let molar solubility of Mg(OH)2 = x = [Mg ]
2+ - Mg(OH)2 Mg + 2OH [initial] ______[] ______[final] ______
Complete :
Example 3:
-10 Ksp for AgCl is 1.1 x 10 . Calculate solubility in moles /L and grams/Litre.
Step 1. Setup Ksp expression.
Step 2. Calculate molar solubility (mol/L).
Step 3. Calculate solubility in g/L.
Complete :
Determine the solubility of AgI, Ag2CrO4, and Ca3(PO4)2 in
o a) moles/L and b) g/L at 25 C. You will have to refer to the Ksp tables in your textbook. 31
Day 9 Problems : Calculating Ksp given solubility
Example 1:
At 200C, it is found that 1.94 x 10-4 g of AgCl saturates 100 ml of water. If the molar mass of AgCl is
143.4 g/mol, calculate Ksp.
Review: concentration = # moles of solution/ Volume of solution
C= ______= ______
Step 1. Find molar solubility in mol/L (i.e concentration) c = n = m = 1.94 x 10-4 g = 1.35 x 10-5 mol/L ------v M x V 143.4 g/mol x 0.10 L
Step 2. Write dissociation equation:
+ - AgCl(s) Ag (aq) + Cl (aq)
H2O
Step 3. Calculate [Ag+] and [Cl-]
[Ag+] = 1 mol Ag+ x 1.35 x 10-5 mol/L AgCl = 1.35 x 10-5 mol/L = [Cl-] ------1 mol AgCl
Step 4. Set up Ksp expression
+ - -5 -5 -10 Ksp = [Ag ][Cl ] = (1.35 x 10 )(1.35 x 10 ) = 1.82 x 10
Alternate solution : when you calculate the molar solubility that is your x value; setup the Ksp expression and substitute in for x. X =1.35 x 10-5 mol/L Ksp = (x)(x) = (1.35 x 10-5)(1.35 x 10-5) = 1.82 x 10-10
Example 2:
0 -3 The solubility of CaF2 at 25 C is 1.70 x 10 g . Calculate Ksp. -----
100 ml H2O
Step 1. [CaF2] =
Step 2. CaF2 ______+ ______
H2O
Step 3. [Ca+2] =
[F-] = 32 Step 4. ksp = =
Example 3:
-4 A saturated solution of Ag2CO3 can be made by dissolving 1.27 x 10 moles of solid Ag2CO3 in 1.00 L -12 of H2O. What is the Ksp for Ag2CO3 ? (Ans: 8.19 x 10 ) + -2 Ag2CO3(s) 2Ag + CO3
1. [Ag2CO3] =
2. [Ag+] =
-2 [CO3 ] =
3. Ksp =
Complete pg 486 (1,3,4b)
Applications : Sparingly Soluble Solutes in Animals:
- proteins and amino acids digested – nitrogenous wastes
- 1. ammonia – water-dwellers – very soluble in water so diffuses into water, very toxic but [ ammonia ] is low 33
- 2. urea NH2 CONH2 - terrestrial animals with no shells – soluble, not very toxic, removed from blood by kidneys – active transport!
- 3. uric acid – terrestrial animals with egg-laying reproduction – almost insoluble and solution is more toxic than urea – ppt out so can’t harm the organism, even the developing embryo
ASSIGNMENT: Ksp PROBLEMS
-8 1. The solubility of PbSO4 in water at 25C is 0.035 g/L. Calculate Ksp for PbSO4. ( 1.3 x 10 )
-3 -12 2. Mg(OH)2 has a solubility of 7.05 x 10 g/L. Calculate Ksp of Mg(OH)2. ( 7 x 10 )
-4 -12 3. The solubility of Ag2CrO4 is 1.32 x 10 mol/L. Calculate Ksp for Ag2CrO4. ( 9.0 x 10 )
-7 -4 4. Ksp of NiCO3 is 1.4 x 10 . Calculate the molar solubility of NiCO3. ( 3.7 x 10 mol/L )
-12 + 5. Given the Ksp for Ag2CrO4 is 1.9 x 10 , what is the concentration in mol/L of Ag ions in a saturated -4 solution of Ag2CrO4? ( 1.6 x 10 mol/L )
-6 6. The solubility of BaF2 is 1.30 g/L. Calculate its Ksp. ( 1.62 x 10 )
7. The formula of a sparingly soluble salt A3B2. The molar mass of A is 40.0 g/mol and B is 30.0 g/mol. If
2.00 g of A3B2 saturates 250 mL of solution, calculate the Ksp for A3B2. ( 1.9 x 10 -5 )
-29 8. The Ksp of Ca3(PO4)2 is 2.0 x 10 . How many grams of Ca3(PO4)2 is required to saturate 2.0 L of solution? ( 4.4 x 10 -4 g ) 34
Read pages 482 -483
Day 10 PROBLEMS: Predicting Precipitate Formation ( pg. 487 ) 2+ -2 - consider the equilibrium: CaCO3(s) Ca + CO3 2+ -2 - for a saturated solution the ion product: [Ca ] x [CO3 ] is exactly equal to ksp.
- precipitate will form if ion product > Ksp. (supersaturated solution) - no precipiate will form if:
ion product = Ksp. (Saturated solution)
ion product < Ksp. (Unsaturated solution)
Example 1: Predicting Precipitate Formation
When the product of the concentration of the ions exceed the value of Ksp they cannot exist in equilibrium anymore. They will form a precipitate in order to reduce the concentrations of the ions in solution back to the equilibrium value.
To determine whether the Ksp is exceeded, just substitute the ion concentrations into an expression similar
to the Ksp expression and determine trial Ksp(Q)
If the trial Ksp. exceeds the Ksp actual then a precipitate forms. If the Ksp trial is larger than the values of Ksp then the ions are above the saturation line and the ions are in a super-saturated situation. The ions will
precipitate until their individual concentrations multiplied together equal the value of Ksp.
If the trial ksp. does not exceed the value of Ksp then the ions are in an unsaturated situation, which means there aren't enough ions dissolved to form a precipitate.
If the trial ksp equals the value of Ksp then the solution is saturated. The solution can hold no more dissolved ions without a precipitate forming.
Problem: Does a ppt. of AgCl form when 1 mL of 0.1 mol/L AgNO3 is added to a beaker containing 1 L of tap water with a Cl- ion concentration of 1.0 x 10-5 mol/L?
- [Cl ]f =______
[AgNO3] f = ______
[Ag+] =
Ksp trial = Q = [Ag+][Cl-] =
-10 The Ksp for AgCl is 1.8 x 10 therefore Ksp trial ______Ksp actual so______ppt. forms.
Ex .2 Does a ppt of AgI form when 10 mL of 0.1 mol/L AgNO3 gets added to 90 mL of a solution -10 -17 containing 1.0 x 10 mol/L of KI? The Ksp for AgI is 8.5x 10
+ [AgNO3] = [Ag ] =
[KI] = [I-] =
Q = = ______
______
Example 3: 35
1.0 ml of 0.02 mol/L of Pb(NO3)2 and 1.0 ml of 0.0040 mol/L of AlCl3 are mixed. Will -5 a precipitate of PbCl2form? [ksp(actual) for PbCl2 is 1.2 x 10 ]
Example 4:
If 50.0 ml of 0.0010 mol/L CaCl2 solution was added to 50.0 ml of 0.010 mol/L Al2(SO4)3, would a -5 precipitate of CaSO4 form? (ksp CaSO4 = 2.4 x 10 )
Step 1.
i) [CaCl2]final = ii) [Ca2+] =
iii) [Al2(SO4)3] =
2- iv) [SO4 ] =
Step 2. Q =
Step 3. Ksp(trial) is Ksp(actual) ______
Complete pg 489 (6)
pg 493 (5,6,7,8,10,12) 36
Review Problems
1. The Ksp for CdS is 6.0 x 10-27. Calculate the molar solubility in mol/L [ 7.8 x 10-14 mol/L ]
-11 -4 2. The Ksp for Mg(OH)2 is 1.0 x 10 . Calculate the molar solubility. [ 1.4 x 10 mol/L ]
-13 -5 3. The Ksp for PbCO3 is 1.0 x 10 . Calculate the solubility in g/L. [ 8.4 x 10 g/L ]
-4 -12 4. The molar solubility of Ag2CO3 is 1.27 x 10 mol/L. Calculate the Ksp. [ 8.19 x 10 ]
-5 5. 1.49g of AgBrO3 dissolves in 1L of water. Calculate the Ksp. [ 3.99 x 10 ]
-8 -36 6. The salt A3B2 has a solubility of 2.5 x 10 mol/L. Calculate the Ksp. [ 1.1 x 10 ]
-4 -5 7. Does a precipitate form when 20.0 ml of 1.0 x 10 mol/L Ba(OH)2 is added to 60.0 ml of 2.4 x 10 -14 -14 mol/L Zn(NO3)2. The Ksp for Zn(OH)2 = 1.8 x 10 [ Ksp(expt) = 4.5 x 10 , ppt forms ]
8. Does a precipitate form when 50.0 ml of 0.0020 mol/L Ca(NO3)2 is added to 100.0 ml of 0.050 mol/L -33 -13 Na3PO4. Ksp for Ca3(PO4)2 = 2.1 x 10 . [ Ksp(expt) = 3.33 x 10 , ppt. forms ] 37
Chemical Energy and Equilibrium (pg 494)
Define :Entropy (S) –
Entropy increases :
1. moles of products in gas state is ______than moles of reactants 2. temperature ______3. change of state from ______to ______to ______
Define :Entropy Change (S) –
- chemical reactions have two natural drives one toward ______enthalpy and one towards ______entropy. If both drives are met in one direction the reaction will be ______i.e. tends to occur on its own.
e.g. Mg(s) + 2 HCl (aq) --> MgCl2 (aq) + H2 (g) + energy
- the forward reaction proceeds toward ______entropy and ______enthalpy thus this is a ______reaction.
- if one drive can be met in one direction and other drive in the other direction the reaction will be in ______
e.g. N2O4 (g) 2 NO2(g)
- the following system is at equilibrium since the drives are met in different directions. Max entropy favors the ______side and max entropy favors the ______side. Thus equilibrium represents a compromise between the drive towards max entropy and the drive toward min enthalpy.
Complete pg 498 (1)
Define : 2nd Law of Thermodymanics - nature is always proceeding to a state of higher entropy.
Determine whether the following reactions show an increase or decrease in entropy.
1.) 2 KClO3 (s) --> 2 KCl (s) + 3 O2 (g)
2.) H2O (l) --> H2O (s)
3.) N2 (g) + 3 H2 (g) --> 2 NH3 (g) 38 4.) NaCl (s) --> Na+1 (aq) + Cl-1 (aq)
5.) KCl (s) --> KCl (l)
6.) CO2 (s) --> CO2 (g)
+1 -1 7.) H (aq) + C2H3O2 (aq) --> HC2H3O2 (l)
8.) C (s) + O2 (g) --> CO2 (g)
9.) H2 (g) + Cl2 (g) --> 2 HCl (g)
10.) Ag+1 (aq) + Cl-1 (aq) --> AgCl (s)
11.) 2 N2O5 (g) --> 4 NO2 (g) + O2 (g)
12.) 2 Al (s) + 3 I2 (s) --> 2 AlI3 (s)
+1 -1 13.) H (aq) + OH (aq) --> H2O (l)
14.) 2 NO (g) --> N2 (g) + O2 (g)
15.) H2O (g) --> H2O (l)
Enthalpy & Entropy
For each of these processes, predict if Entropy increases or decreases.
1. 2H2(g) + O2(g) ⇄ 2H2O(g)
2. 2SO3(g) ⇄ 2SO2(g) + O2(g)
+ - 3. Ag (aq) + Cl (aq) ⇄ AgCl(s)
4. Cl2(g) ⇄ 2Cl(g)
5. H2O(l) ⇄ H2O(g)
6. CaCO3(s) + 180 kJ ⇄ CaO(s) + CO2(g)
7. I2(s) + 608 kJ ⇄ I2(aq)
8. 4Fe(s) + 3O2(g) ⇄ 2Fe2O3(s) + 1570 kJ
Consider both Enthalpy and Entropy and determine if each reaction will a) go to completion b) not occur or c) go to equilibrium
9. H2O(l) ⇄ H2O(g) DH = 150 kJ
10. CaCO3(s) + 180 kJ ⇄ CaO(s) + CO2(g)
11. I2(s) + 608 kJ ⇄ I2(aq)
39 12. 4Fe(s) + 3O2(g) ⇄ 2Fe2O3(s) ∆H = -1570 kJ
13. Cl2(g) ⇄ 2Cl(g) DH = +26.8 kJ
+ - 14. Ag (aq) + Cl (aq) ⇄ AgCl(s) + 86.2 kJ
Consider both Enthalpy and Entropy and determine if each reaction will
a) have a large Keq b) have a small Keq c) have a Keq about equal to 1
15. H2SO4(aq) + Zn(s) ⇄ ZnSO4(aq) + H2(g) DH = -207 kJ
+ - 16. NH4NO3(s) ⇄ NH4 (aq) + NO3 (aq) DH = +30 kJ
17. N2(g) + 3H2(g) ⇄ 2NH3(g) + 92 kJ
18. H2O(l) + 150 kJ ⇄ H2O(g)
19. Ca(s) + 2 H2O(l) ⇄ Ca(OH)2(aq) + H2(g) DH = -210 kJ
Day 11 Experiment Ksp of Calcium oxalate (pg 517)
Independent research :
- Assess the impact of chemical equilibrium processes on various biological, and technological systems ( e.g remediation in areas of heavy metal contamination, development of gallstones, use of buffering in medications, use of barium sulphate in medical diagnosis. Heavy metals such as copper, lead and zinc can accumulate to toxic levels in the human body. A process called chelation which causes a chemical reaction involving an equilibrium shift, removes the metals from the body before permanent organ damage occurs. Sample questions :
1. Why are headache tablets buffered? ______
2. Why is barium sulphate safe to use for X-rays of the digestive system even though barium ions are 40 poisonous. ______
3. How do kidney stones form? ______4. Read article no sweat pg 457. summarize what you learned
______
- Complete pg 522 ( 1-18) ( omit 7,8 )pg 523 (1,2,3, 5,6,10,11,12,13,14,15a,16,17,18,19,20,24,25) 41 Day 12ACID AND BASES (ionic equilibrium)(pg.528)
Arrhenius Concept :
Acids : produce ______ions in solution Bases : produce ______ions in solution
Bronsted - Lowry Theory (S.H. Bronsted and T.M. Lowry - 1923)
ACID - a substance that can donate a ______[Hydrogen ion (H+)] to a base. BASE - a substance that can accept a ______from an acid.
+ - e.g. HCl + NH3 NH4 + Cl ACID BASE Conjugate Acid Conjugate Base (proton donor) (proton acceptor)
Conjugate Base - remove a ______from the acid and add a ______charge. Conjugate Acid - add a ______to the base and add a ______charge.
Amphoteric substances :
- can act as both acids or bases depending on the conditions :
HCl (g) + H2O(l) ------> ______+ ______
NH3(g) + H2O(l) ______+ ______
- -2 + HS (g) + H2O(l) S + H3O ______
-2 - - S + H2O(l) HS + OH ______
Exercise 1
Complete the following acid - base equilibriums : identify acids, bases and conjugate pairs.
1. HNO2(g) + NH3(l) ______+ ______
2. HF(aq) + Cl- (aq) ______+ ______
- - 3. HSO4 (aq) + CN (aq) HCN + ______
4. NH3(aq) + HF(aq) ______+ ______
Neutralization Reactions : Acid + Base ------> Salt + Water
HCl(aq) + NaOH(aq) ------> ______+ H2O(l)
+ H3O + _____ + ______+ ______------> ______+ ______+ 2 H2O(l) 42 Net reaction : ______+ ______------> ______
+ - + - -14 In pure water : [ H3O ] = [ OH ] Ke = Kw = [ H3O ] x [ OH ] = 1.0 x 10
+ - -14 Let x = [ H3O ] = [ OH ] Kw = (x)(x) = 1.0 x 10 x = ______
- H2O is a weak electrolyte -----> poor conductor of electricity due to low concentration of ions in solution
+ - - Consider the equ. : 2 H2O + energy H3O + OH + - or H2O + energy H + OH
+ - - As the temperature increases equilibrium shifts to the ______and the [ H ] and [ OH ] o -14 + - ______; thus Kw ______e.g. at 50 C Kw = 5.5 x 10 [ H ] and [OH ] both equal ______; the solution will be ______( acidic, basic or neutral )
Complete pg 532 (1)
The pH Scale (pg.540) Every aqueous solution is either acidic, basic or neutral. There is a quantitative relationship between the concentration of hydronium and hydroxide ions in the solution. + - Neutral solution [H3O ] = [OH ] + - Acid solution [H3O ] > [OH ] + - Basic solution [H3O ] < [OH ] The brackets as usual denote molar concentrations. The pH scale is a numerical scale which, for most applications extends from 0 through to 14. The numbers on the scale represent the relative acidity of solutions and can be converted into actual hydronium ion concentrations. The pH scale is based on the self-ionization of pure water. Two water molecules will sometimes combine into hydronium and hydroxide ions. + - H2O + H2O <=====> H3O + OH
Pure water is considered to neutral and the hydronium ion concentration is 1.0 x 10-7 mol/L which is equal to the hydroxide ion concentration. + - -7 ie. [H3O ] = [OH ] = 1.0 x 10 mol/L The scale reaches a maximum at 14. Please note again that the hydronium and hydroxide concentrations multiply out to 10-14 M. The pH scale was derived around this relationship: + -pH ie. [H3O ] = 10 mol/L. So the pH is the -log of the [hydronium ion]. See page 541 for calculator tips.
Strong acids and strong Bases
- strong acids are completely ionized in water e.g HCl
Ex.1 What is the pH of 0.15 mol/L HCl HCl ----- H+ + Cl- What is [OH-] ? [i] 0.15 0 0 [c] -.15 +.15 +.15 [e[ 0 +.15 +.15 43 + + [H ] =.15 pH = -log10[H ] = -log100.15 = ______
[OH-] = Kw/[H+] = ______= ______
- Ex. 2 200.0 mL of 0.10 mol/L H2SO4 is diluted to 1.0 L. Calculate pH and [OH ]
Ex. 3 The pH of a 100.0 mL solution of HNO3 is 2.2. Calculate the [HNO3]
[H+] = 10-pH = 10-2.2 = ______
[HNO3] = ______
Strong Bases - completely dissociated in solution
H2O e.g. NaOH ------ Na+ + OH-
The pOH scale is the corollary of the pH scale. ie. pH + pOH = 14
Thus a solution that has a pH = 7 must also have a pOH = 7.
a. What is the pOH of a 0.010 mol/L NaOH solution?
- - [OH ] = ______pOH = -log10[OH ] = ______= ______
b. What is it's pH? ______
- Ex. 2 25.0 g of Ba(OH)2 in 500.0 mL of water. Calculate pOH, pH and [OH ]
i. [Ba(OH)2] = ______= ______
ii. [OH-] = ______
iii. pOH = ______= ______
iv. pH = ______= ______
v. [H+] = ______= ______
Complete pg 540 ( 8,10) pg 546 (12c,13,14) pg 549(18,19) 44
Day 13Weak Monoprotic Acids(pg 551)
- partially ionized in solution
The general equation for a weak acid is: + - HX + H2O H3O + X
+ - + - Applying the equilibrium law to this equation we get: Ka = [H3O ][ X ] [H ][X ] ------= ------[ HX ] [HX]
Ex. 1 (a) Calculate the [H+], (b) the pH and (c) the % ionization for a 0.100 mol/L solution of acetic o -5 acid at 25 C, Ka for CH3COOH is 1.8 x 10 . The equation for the dissociation is: + - CH3COOH H (aq) + CH3 COO (aq) Let 'x' be the concentration of acetic acid that dissociates: + - CH3COOH H (aq) + CH3 COO (aq) [i] ______[c] ______[e] ______
Ka =
x= ______[H+] = ______pH = ______
The % ionization is ______x100% = ______% 0.100 mol/L Note x can be neglected if the % ionization is less than 5%
Ex. 2 Given the equation : HF H+ + F- . Calculate the Ka and % ionization if 0.15mol/L HF has a pH of 2.00 HF H+ + F- [i] ______
[c] ______
[e] ______
Step 1 Calculate [H+]
Step 2 Complete chart
Step 3 Set up Ka expression and solve for Ka 45 Step 4 Calculate % ionization
Complete page 554 (1) pg 556(3) pg 568 (7) pg 570(10)
Weak Bases ( pg 557 ) Similar calculations are used for weak bases. + - eg. NH3(g) + H2O(l) <------> NH4 (aq) + OH (aq)
Kb = ______
The dissociation constant for any of the conjugate bases can be obtained from the value of Kw divided by
the appropriate value of Ka. (Ka can also be obtained by dividing Kw by Kb).
-14 + -10 eg. NH3 Kw = 1.0 x 10 Ka for NH4 = 5.6 x 10
-14 -5 Kb = 1.0 x 10 = 1.8 x 10 5.6 x 10-10
1. What is the [OH-] of a 0.10 mol/L solution of NaCN? Calculate pH. Ka HCN = 6.2 x 10-10 When NaCN dissolves in water it dissolves completely because it is a sodium salt.
NaCN ------> Na+ + CN- 0.1mol/L 0.1mol/L 0.1mol/L
However the CN- ions that are produced then react with water and set up an equilibrium.
- - CN (aq) + H2O <------> HCN(aq) + OH [i] ______
[c] ______
[e] ______
Kb = ______
X = ______= [OH]
-5 Ex. 2 Calculate the pH of a 0.10 mol/L NaCH3COO solution? (Ka CH3COOH = 1.8 x 10 )
+ - NaCH3COO ------> Na + CH3COO
- - CH3COO + H2O CH3COOH + OH 46
Complete pg 574(13) pg 579(3,5,6,13,16,17,18) 47 Assignment Ka and Kb
+ -3 1. The [ H ] of a 0.10 M solution of a weak acid HF is found to be 8.2 x 10 ml/L.
+ - HF + H2O H3O + F
a) What is the [ F- ]? ( 8.2 x 10-3 mol/L )
+ - b) Write the Ka expression. [Ka = [ H3O ] [ F ] ] ------[ HF ]
c) Calculate the Ka and % ionization. ( 6.7 x 10-4 , 8.2% )
2. The initial concentration of an acid HA is 0.0010 mol/L. The pH is 4.0. Calculate the Ka. ( 1 x 10-5 )
3. 0.010 moles of an acid HA is dissolved in a volume of water giving 1000.0 mL of solution. The pH of the solution was 4.0. Determine the Ka ( 1.0 x 10-6 )
+ -5 -3 4. What is the [ H ] in a 0.10 mol/L CH3COOH. The Ka = 1.8 x 10 ( 1.34 x 10 mol/L )
5. A 2.4 g sample of acetic acid was dissolved in 2.00 L of water. The [ H+ ] = 6.0 x 10-4 M. Calculate Ka and
pH of CH3COOH. - + -5 CH3COOH CH3COO + H ( Ka = 1.8 x 10 , pH = 3.2 )
-5 + - 6. Kb for NH3 is 1.8 x 10 . Calculate [ H ], [ OH ], pH, pOH and the percent ionization of a 0.020mol/L NH3 solution. + - NH3 + H2O NH4 + OH
( [ H+ ] = 1.58 x 10-11, [ OH- ] = 6 x 10-4, pH = 10.8, pOH = 3.2, 3% )
-6 7. Kb = 1.7 x 10 for N2H4. If a solution of N2H4 has a pH of 10.5 what is [ N2H4 ] in the solution?
+ - -2 N2H4 + H2O N2H5 + OH ( 5.8 x 10 mol/L )
-4 8. What is the pH of a 0.01 M solution of NaNO2?. The Ka of NaNO2 is 5.1 x 10
NaNO2 ------> + ______
- NO2 + H2O + ( pH = 7.7 ) 48
Read pg 591 Applications Day 14Volumetric Analysis (pg 595)
In many acid-base reactions, the equilibrium is displaced almost completely toward the product side. These reactions may be considered quantitative and can be used as the basis for the analysis of the amount of acid or base in a given sample. The process is termed volumetric analysis. The requirements are:
1) Only a single, specific reaction must take place between the unknown substance and the known substance used for the analysis. 2) The unknown substance must react completely and rapidly with the added standard reagent. ie; it must be a quantitative reaction. 3) An indicator or method must be available to signal when all the unknown substance has reacted with the added standard reagent.
The usual objective is to determine the mass or percentage of a qualitatively identified component in a sample whose quantitative amount is unknown. If the sample is a solution, the objective may be to determine its molar concentration.
A 0.660 mol/L NaOH solution is used to determine the molar concentration of H2SO4 solution. What is the molar concentration of the acid, 20.0 mL of which is just neutralized by 36.0 mL of the standard base? This is a standard problem which can be solved using:
- NaCaVa =Nb CbVb where Na and N b are the # of moles of H+ and # moles of OH as taken from their formulas;
C is the molar concentration, V is the volume, a is 'of the acid', b is 'of the base'.
Therefore : 2 mol x Ca x 20.0 mL = 1 mol x 0.660 mol/L x 36.0 mL
Ca = ______
Complete :
1. What is the molar concentration of a hydrochloric acid solution, 30.0 mL of which is just neutralized by 48.0 mL of 0.100 mol/L NaOH?
2. How many mL of 0.100 mol/L HCl are required to neutralize 25.0 mL of 0.100 mol/L Ba(OH)2?
Standard Solutions To make up a standard solution you usually add a liquid solution of unknown concentration to a solid that has been accurately weighted.
eg. An HCl solution is standardized using pure Na2CO3(mm=106.00) as a primary standard. What is the molar concentration of the acid if 30.00 mL of the acid solution is required to react
completely with a 0.500 gram sample of Na2CO3?
Na2CO3(s) + 2 HCl(aq) ------> 2 NaCl(aq) + H2O(l) + CO2(g)
-3 moles of Na2CO3 = 0.500 grams = 4.72 x 10 moles ------49 106.00 g/mol
Reaction works on a 1:2 basis 1 Na2CO3 = 2 HCl ------4.72 x 10-3 x
x = 9.44 x 10-3 moles of HCl
C = ______= ______
Alternate Solution
NaCaVa =Nbmb _____ Mb
Example 2
A sodium hydroxide solution is standardized by reaction with benzoic acid, HC7H5O2, (M=122.00 grams/mole). A 2.00 gram sample of benzoic acid required 35.00 mL of NaOH to reach the endpoint. What is the molar concentration of the base? Benzoic acid is monoprotic)
Acid-Base Titration (pg 595)
An acid-base titration is just a method by which we can perform a volumetric analysis. The concentration of an acid-base solution may be determined by measuring the volume of the base of known concentration needed to react completely with a specific volume of an acid solution.
Definitions
Standard solution: A solution of ______concentration. Titration: the process of adding the standard solution(titant) from a graduated tube in controlled amounts. Buret: a graduated tube with a dispensor control at the bottom. Equivalence Point: the point at which equal molar quantities of reactants are present. Chemical Indicators: a chemical that will change colour at or very near the ph of the stoichiometric point. Endpoint: The place in the titration when the indicator changes colour.
Titration Curves
A. Strong Acid and a Strong Base 50
Calculate pH : a. no base added [HCl] = 0.300 mol/L --- [H+] = ______pH = ______
b. 5.00 mL of 0.300mol/L NaOH is added
- [NaOH]f = c2 = c1v1 [OH ] = ______------
v2
+ [HCl]f = c2 = c1v1 [H ] = ______------
v2 + [H ]excess = ______pH = ______= ______
c. 20.0 mL of 0.300 mol/L NaOH is added
- [NaOH]f = ______= ______[OH ] = ______
+ [HCl]f = ______= ______[H ] = ______
+ - - + -. the H is consumed completely by the OH forming water. H2O H + OH
Kw = (x)(x) = 1x 10-14 x = 1x 10-7 = [H+] = [OH-] pH = ______=______
d. 30.0mL of 0.300 mol/L NaOH is added
- [NaOH]f = ______= ______[OH ] = ______
+ [HCl]f = ______= ______[H ] = ______
- [OH ]excess = ______pOH = ______pH = ______
Titration of a Weak Acid by a Strong Base 51
At the equivalence point, the solution contains sodium acetate, which has an anion, but not a cation, that hydrolyses. The acetate ions however do ionize to give a slightly basic solution. For this reason, at the equivalence point where all the acid has neutralized all the base, there is still the acetate ions in solution which ionize the water.
-5 a. Initial pH ---> 20.0mL of 0.300 mol/L CH3COOH Ka = 1.8 x 10
- + CH3COOH CH3COO + H [I] [C] [E] Ka = 1.8 x 10-5 = ______
[H+] = ______pH = ______
b. 10.0 mL of 0.300 mol/L NaOH is added
NaOH(aq) + CH3COOH(aq) NaCH3COO(aq) + H2O (l) [i] ______0
[c] ______
[e] ______
+ - + - Na + OH + CH3COOH Na + CH3COO + H2O
- - CH3COO + H2O CH3COOH + OH
[i] ______
[c] ______52 [f] ______
Kb = ______= ______
[OH-] = ______pOH = ______pH = ______
c. after 20.0 mL of 0.300 mol/L NaOH has been added
NaOH(aq) + CH3COOH(aq) NaCH3COO(aq) + H2O (l)
[i] ______0
[c] ______
[e] ______
+ - + - Na + OH + CH3COOH Na + CH3COO + H2O
- - CH3COO + H2O CH3COOH + OH
[i] ______
[c] ______
[e] ______
Kb = ______= ______
[OH-] = ______pOH = ______pH = ______
d) after 30.0 mL of 0.300 mol/L NaOH has been added
Titration of a Weak Base by a Strong Acid + At the equivalence point, when all the acid has neutralized all the base there will still be the cation NH4 in solution. This ion will cause the water to ionize and force the resulting pH of the solution downwards. 53
a. Initial pH with no acid added
+ - NH3 + H2O NH4 + OH [i] _____ 0 0 [c] ______[e] ______
Kb = ______= ______
[OH-] = ______pOH = ______pH = ______
b. After 20.0 mL of 0.100 mol/L HCl is added
NH3 + HCl ______+ ______[i] ______[c] ______[e] ______
+ + NH4 NH3 + H [i] ______[c] ______[e] ______
Ka = ______= ______
[H+] = ______pH = ______
c. After 30.0 mL of 0.100 mol/L HCl is added
54
Try These
1. Calculate the pH of the resulting solution after 20.00 mL of 0.20 mol/L NaOH has been added to 25.00 mL -5 of 0.20 mol/L HC2H3O2. (Ka CH3COOH = 1.8 x 10 )
2. Calculate the pH of the resulting solution after 10.00 mL of 0.20 mol/L NaOH has been added to 25.00 mL + -10 of 0.20 mol/L NH4Cl. (Ka NH4 = 5.6 x10 )
Complete pg 607 ( 5) pg 614(6,9)
Day 15Titration of a Weak Acid
In this activity, you will standardize a sodium hydroxide solution, then determine the concentration of an unknown acid by titration with the standardized base. You will then determine the pH of the acid using a probe and discuss the differences.
Experimental Design This is a two-part activity. In the first part, you will standardize a sodium hydroxide solution by titrating it with the 55 primary standard, potassium hydrogen phthalate, KHC8H4O4(aq) . The concentration of the unknown acid solution is then determined by titrating it with the standardized sodium hydroxide solution.
Materials lab apron KHC8H4O4(s) eye protection 1% phenolphthalein sheet of blank white paper vinegar, lemon juice, or other acid solution of unknown electronic balance concentration pH meter dropper distilled water stirring rod wash bottle with distilled water buret 125-mL Erlenmeyer flask buret stand 1000-mL glass or plastic bottle 100-mL graduated cylinder rubber stoppers 10-mL graduated cylinder NaOH(s) weighing paper
Procedure Part I Standardization of NaOH(aq) 1. In a 125-mL Erlenmeyer flask, dissolve approximately 10 g of NaOH(s) in 50 mL of distilled water. 2. Transfer the entire solution to a 1000-mL bottle and dilute with 500 mL distilled water. Stir the solution but do not shake it. Assume volumes are additive.
3. Weigh approximately 0.4 g KHC8H4O4(s) and record the mass to three significant digits.
4. Place the KHC8H4O4(s) into a clean, dry Erlenmeyer flask. Add 50.0 mL distilled water and two to three drops of phenolphthalein. Swirl to mix. 5. Allow several millilitres of the NaOH(aq) solution to flow through a buret, making sure that the solution wets all of the inside surfaces. 6. Fill the wetted buret with the NaOH(aq) solution and record the volume in a suitable chart.
7. Place the Erlenmeyer flask containing KHC8H4O4(aq) over a sheet of white paper and titrate with the NaOH(aq) solution in the buret until the endpoint is reached. 8. Repeat steps 3 to 7 two more times and calculate the mean of the three volumes of NaOH(aq) used to reach the endpoint. Rinse out the flask.
Part II Determining the [H+(aq)] in a solution of unknown concentration 9. Refill the buret you used in Part A with standardized NaOH(aq) solution. 10. Place 10.00 mL of an unknown acidic solution into a clean, dry Erlenmeyer flask. Add 2 to 3 drops of phenolphthalein. Swirl to mix. 11. Place the Erlenmeyer flask containing the acid solution over a sheet of white paper and titrate with standardized NaOH(aq) solution in the buret until the endpoint is reached. 12. Repeat steps 10 to 11 two more times, and calculate the mean of the three volumes of NaOH(aq) used to reach the endpoint. Use this average value to calculate the concentration of H+ (aq) in the acidic solution. 13. Calibrate a pH meter and use it to measure the pH of a sample of the unknown acid solution. Record the value. 14. Discard all solutions in the sink with lots of running water. Return materials and equipment to their proper location. Wash your hands with soap and water.
Analysis Part 1 (a) Use the average of your titration volumes to calculate the exact concentration of the NaOH(aq) solution. (b) Theoretically (through calculation), what should have been the concentration of the acid (c) What was the pOH and pH of the basic solution?
(d) Why was KHC8H4O4(s) used as the acid in the standardization titration?
Part II (d) Use the evidence from your titration to calculate the concentration of the vinegar solution. (e)Calculate the pH of the acidic solution (need to find Ka of acetic acid) (f) Calculate the % error based on the reading of the calibrated pH meter. 56 (g) After the addition of 10mL of NaOH, what was the approximate pH of the sample solution?
Part III Evaluation (g) Comment on the reasons for the difference in the actual and applied results (sources of error) and evaluate the Procedure and suggest changes that might correct any sources of error.
**For write up of this lab, it is required that you
a. Develop an appropriate question to be answered. b. Develop an appropriate hypothesis, including dependant and independent variables. c. Develop the procedure as a flow chart d. Present the data tables appropriately e. Clearly show your calculations f. Communicate effectively the evaluation g. Develop an appropriate conclusion 57
LAB REPORT RUBRIC Names ______CATEGORIES LEVEL 1 LEVEL 2 LEVEL 3 LEVEL 4 (50-59%) (60-69%) (70-79%) (80-100%) Question/Hyp othesis Question Generating Question is unclear. Question is stated without Question is stated Question is stated clearly using using appropriate scientific using appropriate appropriate scientific terminology. scientific terminology terminology. but is not clear. Hypothesis and Unreasonable association Somewhat reasonable Reasonable association Insightful association between Controls between problem and association between the between the problem the problem and the predicted predicted results, and variables problem and the predicted and the predicted results; all variables are clearly not defined correctly. results, or some variables results, and most key defined. improperly defined. variables are defined.
Procedure Procedural Planning Develops disorganized or Develops an appropriate Develops an Develops an appropriate unworkable procedures. incomplete set of appropriate complete complete set of procedures that Includes few of the important procedures which may set of procedures that are complete, efficient and clear. steps. lack efficiency or clarity. are mostly efficient Includes all of the important Includes some of the and clear. Includes steps. important steps. most of the important steps. Procedure Format Procedure included but not Some procedure steps Procedure written Procedure written completely in written in past tense, written in past tense, mostly in past tense, past tense, impersonal and impersonal and sequentially impersonal and impersonal and sequentially numbered. numbered. sequentially numbered. sequentially numbered.
Observations Data Recording Records little data; data is Records data but Records most relevant Records all relevant data in an irrelevant and/or inaccurate. organization is lacking; data in an organized organized and skillful way; some inaccuracies. way; adequate detail accurately. and generally accurate. Observations Makes few observations Makes observations but Makes sufficient Makes insightful observations may be insufficient to observations to but does not draw conclusions. generate data. generate data.
Analyzing & Interpreting Analysis of Data Provides no analysis of the Explains data but provides Provides some analysis Provides insightful analysis of data. Identifies few patterns or limited analysis. Identifies of the data. Identifies the data. Identifies both obvious trends. some obvious patterns or most of the obvious and subtle patterns and trends. trends. patterns and trends. Analysis questions Answers few questions and/or Answers some questions Answers most Answers all questions part I explanations are incorrect. successfully; explanations questions successfully; successfully; all explanations are are lacking. a few explanations are accurate and thorough. lacking. Analysis question Calculations are done poorly, Calculations are done fairly Calculations are done Calculations are done well, with part II with little information well, with most well, with most all information presented in a presented properly and information presented information presented neat and organized manner with incorrect significant digits. properly with the proper properly with the the proper number of significant number of significant proper number of digits. digits. significant digits. Analysis question Provides no evaluation of Provides limited evaluation Provides some Provides a thorough evaluation part III procedure used. Identifies few of procedure. Identifies evaluation of of the procedure used. Identifies sources of error or limitations. some sources of error or procedure used. all sources of error and limitations. Identifies most of the limitations. sources of error or limitations. Conclusions Makes illogical or irrelevant Attempts to draw some Draws some valid Draws clearly stated conclusions conclusions. conclusions partially based conclusions based on based on the data; relevant and on the data; not clearly the data; relevant and clearly stated. stated. clearly stated. Format Few requirements for Some requirements for Most requirements for All requirements for format/style format/style followed. format/style followed. format/style followed. followed. Scientific Frequent errors in the use of Some errors in the use of Minor errors in the use No errors in the use of units and 58 Terminology & SI units and terminology; units and terminology; do of units and terminology. Units interferes with communication. not interfere with terminology. communication.
Day 16 Buffer Solutions ( pg 615 )
Buffers are solutions with the ability to resist the addition of strong acids or strong bases, within limits. They play an important role in chemical processes where it is essential that a fairly constant pH is maintained. In many industrial and physiological processes, specific reactions occur at some optimum pH value. When the pH varies to any extent from the optimum value, undesirable reactions and effects may occur. For example, the pH of your blood lies at about 7.35. If this value drops below 7.0 (acidosis) the results are fatal. Also if it rises above 7.7 (alkalosis) the results are as well fatal. Fortunately our blood contains a buffering system which maintains the acidity at the proper level. If it were not for the protection of the buffering system, we could not eat and adsorb many of the acidic fruit juices and foods in our diet.
A typical lab buffer is CH3COOH and its salt NaCH3COO. Most buffer solutions are made up using a weak acid and its sodium salt! When a strong base such as NaOH is added to the buffer, the acetic acid reacts with and consumes - - + the excess OH ion. The OH reacts with the H3O ion from the acid in the following reaction: + - H2O + CH3COOH <------> H3O + CH3COO + - H3O + OH <------> H2O - + - The OH reduces the H3O ion concentration, which causes a shift to the right, forming additional CH3COO and + - H3O ions. For practical purposes each mole of OH added consumes a mole of CH3COOH and produces a mole of - CH3COO . - - + OH + CH3COOH <------> CH3COO + H3O - When a strong acid such as HCl is added to the buffer, the hydronium ions react with the CH3COO ions of the salt and form more undissociated CH3COOH. + - H3O + CH3COO <------> CH3COOH + H2O As you would expect, there is a limit to the quantity of H+ or OH- that a buffer can absorb without undergoing a significant change in pH. If a mole of HCl is added to a litre of buffer solution containing 0.5 moles of sodium acetate/acetic acid buffer the H+ completely consumes the buffer and results in a drastic change in pH. The blood buffer is made up from the dissolved carbon dioxide in the plasma. - + CO2(g) + H2O <------> H2CO3 <------> HCO3 + H3O
When a base is added it reacts with the carbonic acid. - - OH + H2CO3 <------> HCO3 + H2O When an acid is added it reacts with the bicarbonate ion. + - H3O + HCO3 <------> H2CO3 + H2O Because there are both a base-neutralizer and an acid-neutralizer then we have a buffer.
Buffer Components
A buffer has two components. HA NaA ---> Na+ + A- a weak acid & a soluble salt of the acid + - Therefore any extra H3O will be neutralized by the A in the buffer.
+ - H3O + A <------> HA + H2O And any extra OH- that is added will be neutralized by the acid. - - HA + OH <------> A + H2O
Day 17 Reading Assignment pg 621-623 : Case Study ( Acid Deposition )
Complete pg 631( 1,2,5,6,7,10-13,16,17,18,19) pg 632(1,5,8,9,(Calculate final pH:15 b,c,f),25) Complete pg 636(1-14,17,19,20,21,22,23-27,32-34,36,39) 59 pg 639(1,2,3,6a,7,9,10,12,16,17,18,19,27,28,30,31,34,44b,f,51,55,56,59,60,62)
SCH 4U1 UNIT 4 REVIEW
1. Consider the equilibrium below: If 1.5 mol of PCl5 was placed in a 1.0 L container and allowed to reach equilibrium, what would the value of Ke be if at equilibrium [PCl5] = 1.2 mol/L? ( ANS 13 ) PCl3(g) + Cl2(g) <=====> PCl5(g)
2. Consider the equilibrium below: If 1.6 mol of HI was placed in a 1.0 L container and allowed to reach equilibrium, what would the equilibrium concentrations be for H2(g), I2(g) and HI(g) if the Ke = 36? H2(g) + I2(g) <=====> 2HI(g) ( ANS . = 0.20 mol/L = [H2] = [I2]; [HI] = 1.6 2(0.20) = 1.2 mol/L )
3. The equilibrium system shown below has a Ke = 1.4 10-4. If [A] = 0.24 mol/L, what is the [D]? 3A(g) + B(s) <=====> C(s) + 2D(g)( ANS [D] = 1.4 10-3 mol/L )
Problem
4. If the solubility of AgBr is 8.8 x 10-7 mol/L, what is its Ksp?(ANS Ksp = (8.8 x 10-7)2 = 7.7 10-13
-4 -12 5. If the solubility of Mg(OH)2 is 1.3 x 10 mol/L, what is its Ksp? ( ANS 8.8 10
-12 6. If 5.6 L of a saturated solution of Ag2CrO4 is found to contain 0.12 g of Ag2CrO4, what is the Ksp of Ag2CrO4. 1.1 10
-5 7. What is the solubility, in mol/L, of MgCO3 in a 0.65 mol/L solution of MgCl2 if the Ksp of MgCO3 is 2.5 10 ? solubility = 3.9 x 10-5 mol/L
-4 8. If 365 mL of a 0.0054 mol/L solution of Pb(NO3)2 was mixed with 595 mL of a 6.34 10 mol/L solution of KI, would a -9 2+ precipitate form? Calculate the ion product for the potential precipitate. The Ksp of PbI2 is 7.9 x 10 . ion product PbI2 is [Pb ] [I1-]2 = 3.2 x 10-10 < Ksp, no precipitate forms
-3 9. If 78 mL of a 0.0026 mol/L solution of Zn(NO3)2 was mixed with 45 mL of a 7.13 x 10 mol/L solution of K2CO3, would a -10 precipitate form? Calculate the ion product for the potential precipitate. The Ksp of ZnCO3 is 1.0 x 10 . ion product ZnCO3 is 2+ 2- -6 [Zn ][CO3 ] = 4.3 x 10 > Ksp of ZnCO3, yes a precipitate occurs
-10 -2 10. What is the concentration of a monoprotic weak acid if its pH is 5.50 and its Ka = 5.7 x 10 ? x = 1.8 10 mol/L
11. A weak base with a concentration of 1.3 mol/L has a percent ionization of 0.72%. What is the Kb of this weak base? Ka = ((0.72)(1.3)/100)2 / 1.3 = 6.7 x 10-5
-9 -3 12. What is the percent ionization of a 0.48 mol/L weak acid if its Ka = 1.4 x 10 ? % Ionization = 100(x / 0.48) = 5.4 10 % 60 -11 13. What is the pH of a 1.47 mol/L solution of HCN(aq) if its Ka = 3.5 x 10 ? pH = 5.14
14. 25 mL of standardized 0.45 mol/L NaOH is titrated with 21 mL of 0.35 mol/L acetic acid. Calculate the pH of the solution. pH = 14 – 1.07 = 12.93 61