The fluctuation of dissolved and particulate trace metals in a small, enclosed, and shallow bay
M. LADAKIS, M. DASSENAKIS AND C. BELIAS
University of Athens, Faculty of Chemistry, Laboratory of Environmental Chemistry Panepistimioupolis 157 71 Athens, GREECE
Abstract The small, isolated and shallow bays of the Greek coastline differ significantly from the open sea in the degree of eutrophication, the fluctuation of hydrological parameters and the concentration of pollutants, like trace metals. Those characteristics can create interesting environmental conditions that may affect trace metals’ behaviour and accumulation to algal mats or to surface sediments. The variations of dissolved and particulate forms of Al, Cd, Cu, Fe, Mn, Ni, Pb and Zn along with the variations of pH, salinity, water temperature and eutrophication degree at a bay near to Athens, are studied in this paper. Monthly water samplings and in situ measurements for pH, salinity and temperature were carried out for a period of one year (July 2000 – June 2001) at two points inside and one outside the under study bay. The results of the various determinations showed that the waters inside the bay are more eutrophicated compared to the open sea, whereas there is no significant difference in the pH, temperature and salinity. The heavy metals’ concentrations of the bay’s water (in both dissolved and particulate form) are directly affected by the exterior marine environment and by freshwater incomes. The determined metal concentrations into the cove are rather favourable than obstructive for the phytoplankton growth. This conclusion in combination with the absence of a limiting factor for the primary production create a favourable environment for the development of a layer of algae mat that extends through the bottom of the bay.
Key-Words: Isolated bays, Algal mat, Particulate metals, Dissolved metals
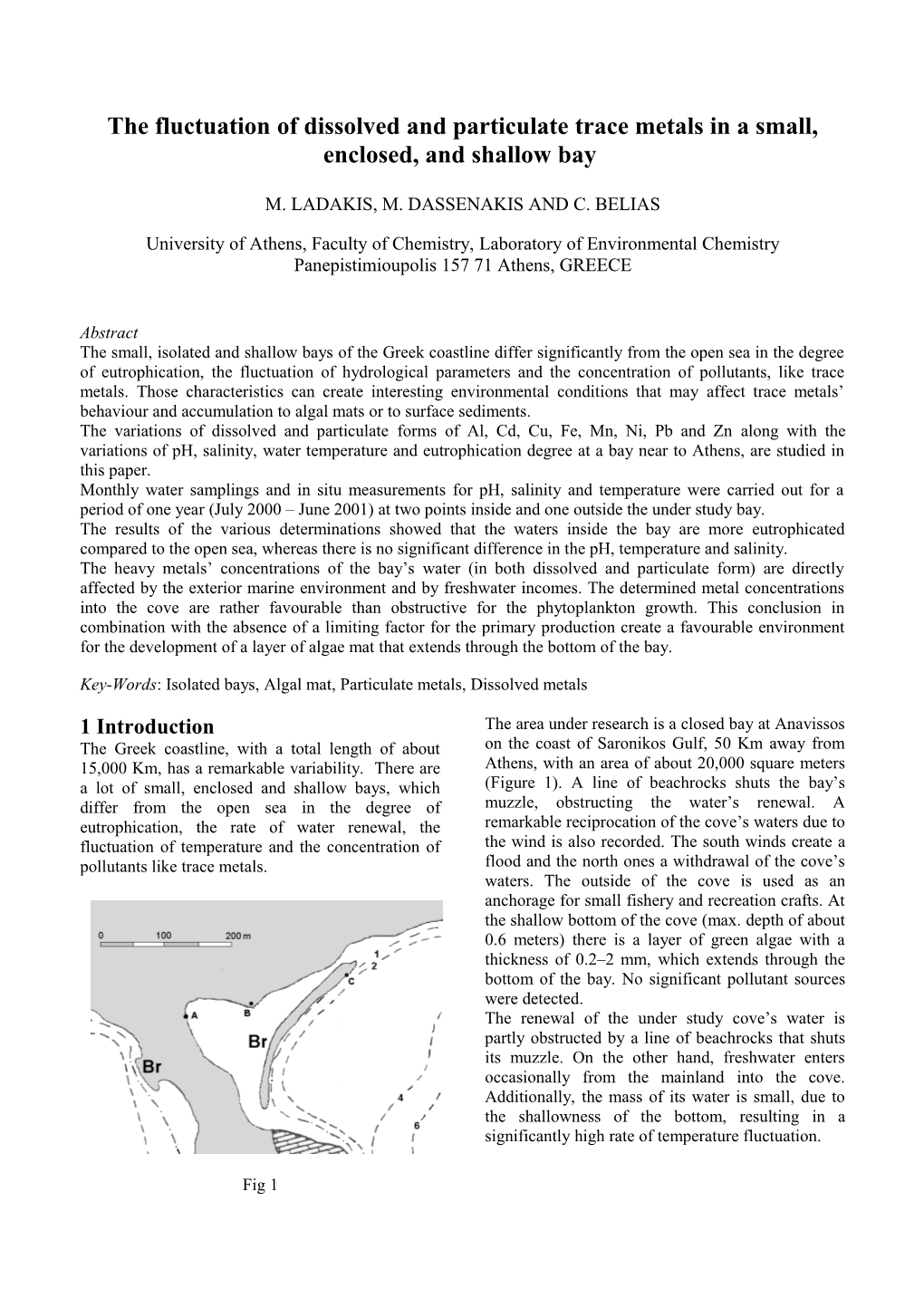
1 Introduction The area under research is a closed bay at Anavissos The Greek coastline, with a total length of about on the coast of Saronikos Gulf, 50 Km away from 15,000 Km, has a remarkable variability. There are Athens, with an area of about 20,000 square meters a lot of small, enclosed and shallow bays, which (Figure 1). A line of beachrocks shuts the bay’s differ from the open sea in the degree of muzzle, obstructing the water’s renewal. A eutrophication, the rate of water renewal, the remarkable reciprocation of the cove’s waters due to fluctuation of temperature and the concentration of the wind is also recorded. The south winds create a pollutants like trace metals. flood and the north ones a withdrawal of the cove’s waters. The outside of the cove is used as an anchorage for small fishery and recreation crafts. At the shallow bottom of the cove (max. depth of about 0.6 meters) there is a layer of green algae with a thickness of 0.2–2 mm, which extends through the bottom of the bay. No significant pollutant sources were detected. The renewal of the under study cove’s water is partly obstructed by a line of beachrocks that shuts its muzzle. On the other hand, freshwater enters occasionally from the mainland into the cove. Additionally, the mass of its water is small, due to the shallowness of the bottom, resulting in a significantly high rate of temperature fluctuation.
Fig 1 2 Problem Formulation The Flame and Graphite Furnace Atomic Coastal areas like the ones described above, Absorption Spectrometry were used for the although small, can have specific interest because determination of both particulate and dissolved the conditions prevailing there differ from the forms of Al, Cd, Cu, Fe, Mn, Ni, Pb and Zn. neighbouring ones. In those environments, the values of 3 Results and Discussion physicochemical as well as the concentration of 3.1 Physicochemical parameters chemical parameters (i.e nutrients, metals, pigments, The variation of the pH was between 7.97 and 8.27 pollutants, e.t.c) are expected to differ in values and at all sampling points. No remarkable difference in temporal fluctuation in comparison to the ones of values between the interior and the exterior side of the adjusted coastal areas. In addition, such the studied area was noted. The seasonal fluctuation environments seem to encourage the development of of pH in all 3 sampling points was similar. algal mat on the surface of the bottom. Salinity ranged between 34.5 and 39.8 in the cove On the other hand, the external marine (points A and B) and between 35.5 and 38.4 in the environment’s influence to the metals concentration external point (point C), with the exception of one at the bay’s water is expected to be considerable. case (March 2001), where salinity was very low in This study deals with the variations of dissolved and both points A and B inside the cove (18.2 and 22.2 particulate metals together with the variations of pH, respectively). This was probably due to freshwater salinity, water-temperature and eutrophication income at this time. Nevertheless, the values of degree at the bay’s water. salinity (represented in figure 2) indicate that there is no permanent freshwater income into the cove. 2.1 Materials, Methods and apparatus Salinity Monthly water samplings and in situ measurements 40,00 of pH, salinity and temperature were carried out for a period of one year (July 2000 – June 2001) at two 35,00 points inside the cove, named point A and point B. 30,00
At the same time, similar measurements and S samplings were taken from a point outside the bay, 25,00 to be used as a reference, named point C. 20,00 point A point B The physicochemical parameters (pH, salinity and point C temperature) were measured by the use of a portable 15,00 Jun- Jul- Aug- Sep- Oct- Nov- Dec- Jan- Feb- Mar- Apr- May- Jun- salinometer which was calibrated with a pH buffer 00 00 00 00 00 00 00 01 01 01 01 01 01 solution before every measurement. months The water samples for nutrient analysis were Fig 2 collected in plastic bottles (pre-treated overnight The water temperature varied between 14.7 (in with 1N HCl solution) and stored in –18oC until the January) and 33.2oC (in July) inside, and between time of analysis. Standard spectrophotometric 14.6 and 26.8oC outside the cove, respectively. methods [1] were used for the determination of During the summer, the water was warmer inside nutrients by the use of a Carry-100 double beam the cove than outside. spectophotometer. The water samples for metal analysis (volume of 2 l 3.2 Eutrophication degree each) were collected in glass bottles (pre-treated The Karidis et al. criteria [3] have been applied to overnight with 2N HNO3) and conserved until the the nutrient concentration values in order to time of analysis by adding a few drops of characterize the area’s eutrophication degree. concentrated HNO3. The annual min, mean and max values of the area’s The samples were filtered through pre-weighted nutrient concentration are presented in table 1. nitrocellulose filters (Milipore 0.45 μm) in order to Table 1. Nutrients concentration (mean values in separate the particulate from the dissolved form of parenthesis – all values in μgion/l) metals. The filters were treated with 40ml 1:1 HNO3 Inside the bay Outside the bay in teflon bakers and after their complete dissolution, [NO3] 0.62 - 2.27 (1.27) 0.58 - 1.82 (1.16) the volume was adjusted to exactly 50 ml. [NO2] 0.04 - 0.13 (0.06) 0.01 - 0.07 (0.04) The dissolved metals were pre-concentrated by [NH4] 0.14 - 1.75 (0.69) 0.02 - 1.47 (0.63) passing the sample through Chelex 100 resin [2] and [PO4] 0.03 - 0.48 (0.14) 0.02 - 0.26 (0.13) eluting them by 50 ml nitric acid 2N.). According to those criteria, the water inside the bay the fluctuation of particulate Cd and Ni in the cove’s was in the Upper Mesotrophic level during 8 of the water, as shown in figures 3 and 4: 12 months of the period, while Lower Mesotrophic level characterize the water outside the bay during 8 of the 12 months of the period. No nutrient limiting factor for the phytoplankton growth was found according to the Justic et al (1995) criteria [4], except for the months October and November, where Phosphorus was the limiting factor.
3.3 Dissolved and particulate metals The studied metals were separated in three groups: a) Pollutants (which include Cd, Ni and Pb), b) Biologically importants (which include Cu, Mn, Fe and Zn) and Fig 4 c) Metals derived from geological sources (which The dissolved form of Pb ranged between 1.45 and include Al and Fe). 10.59 μg/l inside the cove and between 0.37 and a) The pollutants 6.51 μg/l outside. The highest values were recorded The dissolved forms of Cd ranged between 0.08 and during the hottest period of the year with the 0.59 μg/l inside the cove and between 0.01 and 0.59 exception of August. μg/l outside. The mean annual values were 0.32 and 0.28 μg/l respectively. A fair correlation in the fluctuation of dissolved Cd inside and outside the cove (r = 0.71 for p < 0.05, statistically important according to the t-test), was detected. Particulate Cd (figure 3) was found to be even lower, as annual mean values were 0.12 and 0.08 μg/l inside and outside the cove respectively. On the other hand, a significantly high value (0.75 μg/l) was recorded during July 2000. Nevertheless, the values of both dissolved and particulate forms of Cd are low for coastal waters. The values of dissolved Ni inside the cove were Fig 5 found to be less than 1.55 μg/l, with the exception of two cases (July 2000 and April 2001 with values of 2.39 and 3.63 μg/l respectively). The mean value outside the cove was 0.95 μg/l.
Fig 6 A good correlation in the fluctuation of dissolved Pb inside and outside the cove (r = 0.88, statistically important according to the t-test) was also detected, Fig 3 as shown in figure 5. A rather random fluctuation The particulate Ni was recorded at the same levels was detected for particulate Pb, as it is indicated by with the dissolved one, both inside and outside the figure 6. cove, with the exception of July 2000, when a In general, the cove’s water is enriched with Pb significantly high value (12.9 μg/l) was recorded in when compared with the water outside. the cove’s water. In general, there is a similarity in b) The biologically importants The fluctuation of the dissolved Cu both inside and outside the cove is presented in figure 7.
Fig 9 Fig 7 c) Metals originated from geological sources A slight increase of its concentration is observed The abundance of particulate Al in coastal waters is during the hot period of the year. The relatively high well known, as it usually originates from the value that was recorded outside the cove in October corrosion of argillaceous minerals. As extracted 2000 (10.3 μg/l) was accompanied by a respectively from figure 10, the fluctuation of particulate Al high value inside the cove (4.64 μg/l). The is random and the concentration inside the cove concentration of the particulate Cu was less than 1 is higher than prevailing in the outside water. μg/l both inside and outside the cove. The concentrations of dissolved Zn ranged between 0.6 and 8.6 μg/l both inside and outside the cove. A remarkable similarity in the monthly fluctuation of Zn inside and outside the cove was recorded (r = 0.71, statistically important according to the t-test). As extracted from figure 8, the dissolved Zn concentration increases during the hottest period of the year and decreases during winter.
Fig 10 A strong correlation between particulate Al and Fe was observed inside the cove (r = 0.94 for p < 0.05), as shown in figure 11, indicating the mineral origin of Fe in the cove’s water.
Fig 8 The concentration of particulate Zn was found to be lower than 2.5 μg/l in most cases and a slight increasing trend during the hot period of the year was noted (figure 9). A peak of particulate Zn was observed during March 2001 in the waters outside the cove and was reflected in the interior waters. The water inside the cove was found to be enriched in dissolved Mn, but, on the other hand, a fair Fig 11 correlation of its monthly fluctuation between those The dissolved metals’ concentration of the under two water masses occurred (r = 0.76 for p < 0.05, study area is much higher, compared to the values statistically important according to the t-test). prevailing in various areas of the open Saronikos A strong correlation between particulate Mn and Fe gulf [8], as extracted from table 2. The specific was observed both inside and outside the cove (r = morphological characteristics and the coastal 0.91 and 0.95 respectively for p < 0.05). character of this area, contribute to elevated values of dissolved metals’ concentration. Table 2. Concentration of dissolved metals at the This conclusion, in combination with the upper open Saronikos gulf (values in μg/l). mesotrophic level of the cove’s water, its Area Cd Cu Μn Νi Pb Ζn shallowness (up to 0.6 m), the high level of sunlight, W. Saronikos gulf 0.05 0.16 0.20 0.33 1.13 3.14 the fluctuation of the water’s temperature -that is S. Saronikos gulf 0.02 0.09 0.12 0.25 0.11 1.29 maintained between 14.7 and 33.2 oC during the E. Saronikos gulf 0.05 0.13 0.16 0.29 0.35 2.53 year- and the absence of a limiting factor for the primary production (photosynthesis) create a Points A & B 0.32 3.13 3.75 1.31 4.71 4.23 favourable environment for the layer of algae mat Point C 0.28 2.80 1.19 1.48 2.85 4.21 development. This mat covers the whole surface of the cove’s bottom and reaches to the upper point of 4 Conclusions the flood. The small and semi-isolated marine ecosystems have special characteristics when compared with References: other coastal areas. They present a high degree of 1. K. Grasshoff, and K. Kremlin: Methods of complexity and sensitivity in physical perturbations Seawater Analysis, III edition, Wiley-VCH and human pollution. Various coastal Torondo 1997, pp 159 – 226 microenvironments, such as the Vouliagmeni lake in 2. Riley, J.P. and Taylor, D. (1968). Chelating resins the peninsula of Attica and the Agios Nikolaos lake for the concentration of trace elements from in east Crete, have also been studied [5,6]. The seawater and their analytical use in conjunction common result extracted is that each with A.A.S. Anal. Chemical Acta, 40, 479 – 484. microenviromnent has its own characteristics. 3. M.Karidis, L. Ignadiades, A Mouriki, G.Tsirtsis The general results for the studied small gulf are: and D. Kitsiou: Quantitative assessment of The salinity decreases occasionally, due to eutrophication: Criteria development for the freshwater incomes from the mainland. management of coastal waters (1988) The metal concentration of the cove’s waters (in 4. D.Justic, N.N. Rabalais and R.E. Turner both dissolved and particulate form) is directly Stoichiometric nutrient balance and origin of affected from the exterior marine environment. eutrophication. Marine Pollution Bulletin 30(1) A good correlation in the fluctuation of some metals (1995), pp 41 – 46. concentration inside and outside the cove occurs 5. M.Scoullos, A.Pavlidou, M.Dassenakis, P.Beza, (dissolved Zn, Pb, Mn and Fe as well as particulate Environmental studies in a complex brakish Zn). system: The Bouliagmeni lake, Greece, Surcharge events outside the cove reflect directly at International Conference on Restoration and a respective high concentration in the internal Protection of the Environment III, Chania, August waters (dissolved Cu in October 2000, dissolved Zn 1996, Proc.pp.149-157 in April 2001, particulate Zn in March 2001, 6. N.Michopoulos, M.Dassenakis, G.Anastasakis, particulate Al in September 2000). M.Scoullos, Physical and chemical characteristics The determined metal concentrations into the cove of a Mediterranean coastal microenvironment, the are more favourable than obstructive for the salty lake of Agios Nikolaos, Crete, Greece. Global phytoplankton growth as extracted from table 3. Nest: the International Journal. Vol 2, No 2, pp 167-178, 2000. Table 3. Normal, favourable and restrictive 7. Skinner B.J., and Turekian K.K., (1973), Man and concentration of some dissolved metals for the the Ocean, Prentice-Hall, Englewood Cliffs, New phytoplankton growth [7]. Jersey. Concentration of metals (μg/l) 8. Med-Pol project for the monitoring of Saronicos Normally at the gulf. Dissolved metals’ concentration data for the favorable restrictive open sea year 2000. [Al] 1,500-2,000 [Cd] 0.11 [Cu] 2 6 - 200 [Fe] 3.4 0.006 - 10 [Mn] 1.9 0.05 - 50 [Ni] 2 [Pb] 0.03 [Zn] 2 6.5 500-5,000