CHM101: Handout for Exam #4 © 2005, Dr. Miroslav Rezac CHM-101: Review for Semester Exam #4 Dr. Miroslav Rezac
Calculations involving chemical equations.
The first type of calculations gives you the amount of one reactant or product and you calculate the “matching” amounts of all other components of the equation. For example, you may be asked the following.
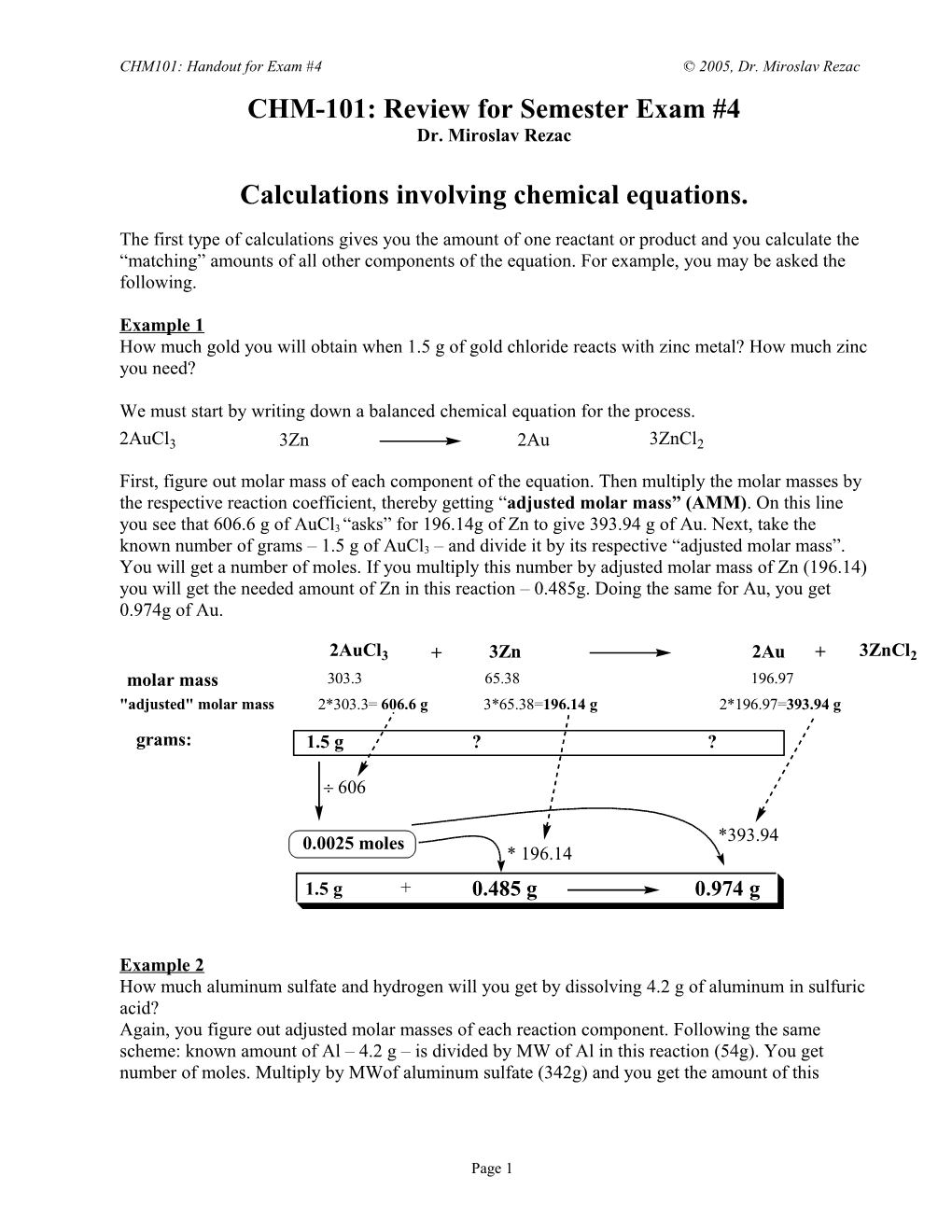
Example 1 How much gold you will obtain when 1.5 g of gold chloride reacts with zinc metal? How much zinc you need?
We must start by writing down a balanced chemical equation for the process.
2AuCl3 3Zn 2Au 3ZnCl2
First, figure out molar mass of each component of the equation. Then multiply the molar masses by the respective reaction coefficient, thereby getting “adjusted molar mass” (AMM). On this line you see that 606.6 g of AuCl3 “asks” for 196.14g of Zn to give 393.94 g of Au. Next, take the known number of grams – 1.5 g of AuCl3 – and divide it by its respective “adjusted molar mass”. You will get a number of moles. If you multiply this number by adjusted molar mass of Zn (196.14) you will get the needed amount of Zn in this reaction – 0.485g. Doing the same for Au, you get 0.974g of Au.
2AuCl3 + 3Zn 2Au + 3ZnCl2 molar mass 303.3 65.38 196.97 "adjusted" molar mass 2*303.3= 606.6 g 3*65.38=196.14 g 2*196.97=393.94 g
grams: 1.5 g ? ?
0.0025 moles *393.94 * 196.14 1.5 g + 0.485 g 0.974 g
Example 2 How much aluminum sulfate and hydrogen will you get by dissolving 4.2 g of aluminum in sulfuric acid? Again, you figure out adjusted molar masses of each reaction component. Following the same scheme: known amount of Al – 4.2 g – is divided by MW of Al in this reaction (54g). You get number of moles. Multiply by MWof aluminum sulfate (342g) and you get the amount of this
Page 1 CHM101: Handout for Exam #4 © 2005, Dr. Miroslav Rezac substance generated in the reaction – 26.7g. Doing the same for hydrogen will give you 0.47g of hydrogen? 3H SO 2Al + 2 4 Al2(SO4)3 + 3H2 molar mass 27 342 2 "adjusted" molar mass 2*27= 54 g 1*342=342 g 3*2=6 g grams: 4.2 g ? ?
0.0778 moles * 342 *6 4.2 g 26.7 g + 0.47 g
************************************ The second type of calculations involves the so-called “limiting reagent”. As opposed to calculating “how much of B will I need to react with 2.5 g of A”, the issue is “if 2g of A react with 4.5g of B, how much products will I get”? As you can see, the issue is whether the amounts of A and B “match”, and if not, to figure out which one is the “weakest link”, as the reagent in excess can only react with the limiting reagent present.
Example 3 Calculate how much of silver chloride you get from reaction of 1.0g silver nitrate with 0.5g sodium chloride. The first task is to figure out AMM’s for each reaction component. Then, divide any known masses of substances (in our case AgNO3, NaCl) by their respective AMMs. This gives you number of moles. Select the smallest of the numbers (5.88*10-3 is less than 8.55*10-3). Use this as a “magic key” to give you the unknown gram amounts: taking adjusted molar masses of remaining components (in this case AgCl) and multiplying them with the “magic key” you get the unknown masses.
AgNO3 + NaCl AgCl + NaNO3 molecules: 1 molecule 1 molecule 1 molecules moles: 1 mol =170.0g 1 mol = 58.5 g 1 mol = 143.4 g grams: 1.0 g 0.5 ? 170 58.5 # of moles: 5.88*10-3 mol < 8.55 *10-3 mol 5.88*10-3 mol use the smaller # * 143.4
0.84g of AgCl
Page 2 CHM101: Handout for Exam #4 © 2005, Dr. Miroslav Rezac
Example 4 “Please determine how much magnesium chloride you get by reaction of 1.0g magnesium and 1.0g of HCl.” Again, the starting point is a balanced equation for the reaction. Then follow the scheme outlined above.
Mg + 2HCl MgCl2 + H2 molar mass 24.3 g 36.5 g 95.2 g
"adjusted" molar mass 1*24.3=24.3g 2*36.5=73g 1*95.2 = 95.2g
grams: 1.0 g 1.0 g ?
24.3 73 # of moles: 41.2*10-3 moles > 13.7 *10-3 moles 13.7 *10-3 moles use the smaller # *95.4
1.30 g of MgCl2
GASEOUS STATE OF MATTER Kinetic Theory
Kinetic theory of matter: please know the following 1. All matter composed of small particles of definite size and mass 2. The particles are in constant random motion and thus have kinetic energy 3. Kinetic energy of particles increases with increasing temperature. Kinetic energy has “disorganizing” effect 4. Particles interact with one another. Attraction is characterized by a potential energy, which has “organizing” effect 5. Collisions between particles are elastic (total kinetic energy is preserved)
As kinetic energy increases with increasing energy, the motion becomes more chaotic and matter more disorganized.
SOLID Strong attractive forces between particles Relatively low kinetic energy – oscillation of particles Definite volume and shape Particles very close together – high density, low compressibility
LIQUID
Page 3 CHM101: Handout for Exam #4 © 2005, Dr. Miroslav Rezac
Attractive forces still relatively strong (although weaker than in solid) Particles freely move throughout the volume – a lot of random motion Indefinite shape; definite volume Particles still very close together - high density, low compressibility
GAS Virtually no attractive forces between particles – each particle “on its own” Fast, independent motion – very high content of kinetic energy Indefinite shape and volume Particles far apart – very high compressibility, low density Solid Liquid Gas Forces btw particles strong strong none Motion minimal random motion chaotic, fast -oscillation throughout motion Defined/stable Shape Yes No No Defined volume Yes Yes No Distance between particles minimal minimal Large
GAS LAW VARIABLES
We already know: Temperature – T (always in Kelvin), Volume - V, Number of moles – n
New one: Pressure – p Pressure can be understood as a force exerted against a unit area. Pressure in the gas is the result of impact of particles on the walls of the container. The more numerous and the higher the speed of impacts the higher the pressure: thus, gas confined into a smaller volume (more numerous) or at higher temperature (higher speed) exerts higher pressure.
SI unit for pressure is 1 Pascal (Pa). Other units is an atmosphere (~105 Pa), and millimeter of mercury (mm Hg), which is identical to a torr. COMBINED GAS LAW
Combined gas law, links T, V and p. It is used when the same gas body (balloon or air inside syringe) is moved from one situation (for example cold temperature) to a new situation (hot temperature).
Combined gas law can be simplified if one of the variables is not “used” (does not change during the experiment). That way you can get the simplified versions. Please do NOT memorize the names.
Page 4 CHM101: Handout for Exam #4 © 2005, Dr. Miroslav Rezac combined gas law p p V V p constant p *V p2*V2 V constant 1 2 1 2 1 1 = = = T1 T2 T1 T2 T1 T2 Guy-Lussac Law Charles's Law T constant
p1*V1 = p2*V2 Boyle Law
p*V p1*V1 p2*V2 = constant OR = T T1 T2
BOYLE LAW
This law states that for the same amount of gas at constant temperature p*V = constant; or p1V1 = p2V2
CHARLES’S LAW
This law states that at constant pressure, for a constant amount of gas, the ratio of volume to temperature stays constant: V V1 V2 = constant OR = T T1 T2
GAY-LUSSAC LAW
This law states that at constant volume, for a constant amount of gas, the ratio of pressure to temperature stays constant:
p p1 p2 = constant OR = T T1 T2
Example 1: Party balloon filled with helium at T = 25 normal pressure p = 100,000 Pa and having a volume of 2 liters. Balloon rises to 10,000 ft where temperature is –20 degrees of Celsius, pressure p=35,000 Pa. What would be the volume? Show solution.
1. Remind to convert Celsius to Kelvin first 2. Draw a little cartoon outlining the situation
3. For both situations list known variables and identify unknown
Page 5 CHM101: Handout for Exam #4 © 2005, Dr. Miroslav Rezac
Ground high altitude P = 100,000Pa p=35,000Pa V= 2L V=x T=298K T=253K
4. Plug values into the combined gas law
"ground" "high up" p *V p *V 1 1 = 2 2 T1 T2
100,000*2 35,000 * X = 298 253
5. Calculate as much as you can on either side – multiply, divide – it greatly simplifies the equation 671.14 = 138.34 * X
6. In this form it is easy to calculate X by dividing the two numbers
X = 671.34 / 138.34 = 4.85 Liters
Example 2: Syringe is filled with 100mL air under pressure 100,000 Pa. Then the opening is closed with thumb and plunger moved to 60mL mark. What is the pressure inside?
1. Convert Celsius to Kelvin first 2. For both situations list known variables and identify unknown
“relaxed” “compressed” P = 100,000Pa p=X V= 100mL V=60mL
3. Temperature is not mentioned and is constant – experiment performed in the same room within seconds 4. Thus, simplified version of gas law is to be used (Boyle law, see above) 5. Plug values into the combined gas law and solve
"relaxed" "compressed"
p1*V1 = p2*V2 100,000*100 = X * 60
10,000,000 = X * 60
X = 10,000,000 / 60 = 166,667 Pa
Page 6 CHM101: Handout for Exam #4 © 2005, Dr. Miroslav Rezac IDEAL GAS LAW
Ideal gas law is used whenever gas law variables (p, V, T) appear with mass (“grams”) in the same problem: p*V = n*R*T
Where R is the “universal gas constant”. Its value depends only on your choice of units; once you chose units its value is independent of which gas you are working with! for V in m3, p in Pa, T in K, n in mol: R = 8.3 J*mol-1*K-1
Note that the gas law does not contain mass directly. Instead, whenever you know mass, you can calculate number of moles: n = m / MW (MW is molar mass).
Example 1: Weather balloon is filled with helium at T = 25 normal pressure p = 100,000 Pa. The balloon has volume V = 2 m3. Point out that this is a sphere around 5 feet in diameter.
1. Remind to convert Celsius to Kelvin first
2. List known variables and identify unknown p = 100,000Pa T = 298 K n = X V = 2 m3
3. Remind that keeping your solution well organized prevents errors
4. Plug values into the ideal gas law, solve each side and calculate X:
p * V = n * R * T 100,000*2 = X*8*298
200,000 = X * 2384
X = 200,000 / 2384 = 83.7 mole
5. Last step is conversion of moles to grams using molar mass (MW); for helium MW = 4 g/mol m = n * MW m = 83.7 * 4 = 335.6 g
Page 7 CHM101: Handout for Exam #4 © 2005, Dr. Miroslav Rezac
Example 2: Vial of volume V=100mL is filled with 2.00 g of liquid chloroform. The vial is sealed and heated to 220 degrees of Celsius in a sterilization unit. At this temperature, all chloroform evaporates. What will be pressure in the vial?
1. Convert into proper units Kelvin, m3
2. Calculate n which corresponds to 2.00g of chloroform: MW for chloroform is 119 g/mol n = m / MW = 2.00 / 119 = 0.0168 moles
3. List known variables and identify unknown T= 493 K V = 1.0*10-4 m3 p = X n = 0.0168 moles
4. Plug into ideal gas law: p * V = n * R * T X* 1.00*10-4 = 0.0168*8*493 X* 1.00*10-4 = 66.26 X = 66.26 / 1.00*10-4 = 662,600 Pa
DALTON LAW OF PARTIAL PRESSURES
This law states that partial pressure of each gas in mixture is equal to pressure the gas would have if it occupied the given volume alone. Sum of all partial pressures is the total pressure. If we know the molar fraction of the gas in question (= # of moles of this gas: total # of moles of all gasses in mixture) we can calculate its partial pressure from total pressure. p = p1 + p2 + p3 etc. p1 = p * (# of moles / total # of moles) p1*V = n1*R*T
Page 8