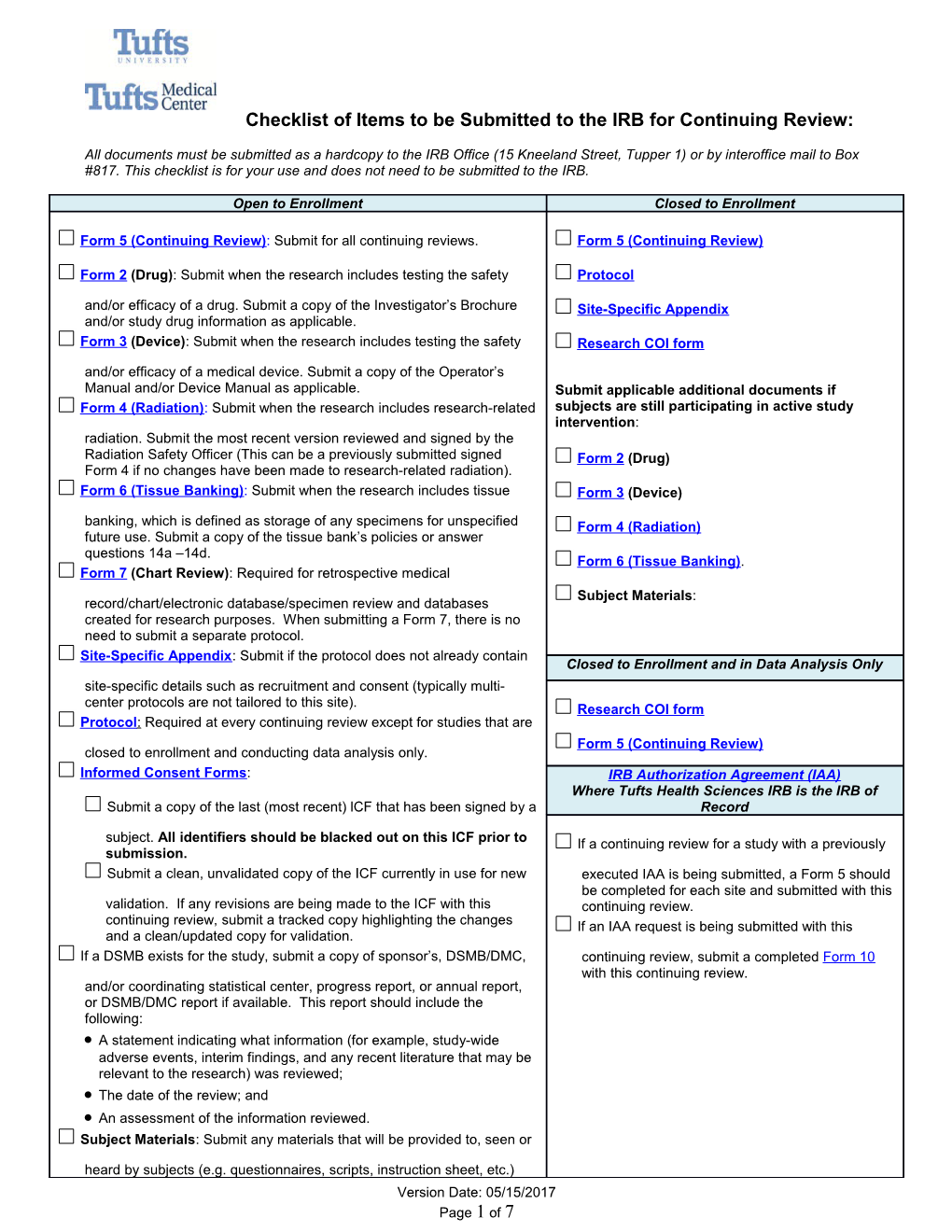
Checklist of Items to be Submitted to the IRB for Continuing Review:
All documents must be submitted as a hardcopy to the IRB Office (15 Kneeland Street, Tupper 1) or by interoffice mail to Box #817. This checklist is for your use and does not need to be submitted to the IRB.
Open to Enrollment Closed to Enrollment
Form 5 (Continuing Review): Submit for all continuing reviews. Form 5 (Continuing Review)
Form 2 (Drug): Submit when the research includes testing the safety Protocol and/or efficacy of a drug. Submit a copy of the Investigator’s Brochure Site-Specific Appendix and/or study drug information as applicable. Form 3 (Device): Submit when the research includes testing the safety Research COI form and/or efficacy of a medical device. Submit a copy of the Operator’s Manual and/or Device Manual as applicable. Submit applicable additional documents if Form 4 (Radiation): Submit when the research includes research-related subjects are still participating in active study intervention: radiation. Submit the most recent version reviewed and signed by the Radiation Safety Officer (This can be a previously submitted signed Form 2 (Drug) Form 4 if no changes have been made to research-related radiation). Form 6 (Tissue Banking): Submit when the research includes tissue Form 3 (Device) banking, which is defined as storage of any specimens for unspecified Form 4 (Radiation) future use. Submit a copy of the tissue bank’s policies or answer questions 14a –14d. Form 6 (Tissue Banking). Form 7 (Chart Review): Required for retrospective medical Subject Materials: record/chart/electronic database/specimen review and databases created for research purposes. When submitting a Form 7, there is no need to submit a separate protocol. Site-Specific Appendix: Submit if the protocol does not already contain Closed to Enrollment and in Data Analysis Only site-specific details such as recruitment and consent (typically multi- center protocols are not tailored to this site). Research COI form Protocol : Required at every continuing review except for studies that are Form 5 (Continuing Review) closed to enrollment and conducting data analysis only. Informed Consent Forms: IRB Authorization Agreement (IAA) Where Tufts Health Sciences IRB is the IRB of Submit a copy of the last (most recent) lCF that has been signed by a Record
subject. All identifiers should be blacked out on this ICF prior to If a continuing review for a study with a previously submission. Submit a clean, unvalidated copy of the ICF currently in use for new executed IAA is being submitted, a Form 5 should be completed for each site and submitted with this validation. If any revisions are being made to the ICF with this continuing review. continuing review, submit a tracked copy highlighting the changes If an IAA request is being submitted with this and a clean/updated copy for validation. If a DSMB exists for the study, submit a copy of sponsor’s, DSMB/DMC, continuing review, submit a completed Form 10 with this continuing review. and/or coordinating statistical center, progress report, or annual report, or DSMB/DMC report if available. This report should include the following: A statement indicating what information (for example, study-wide adverse events, interim findings, and any recent literature that may be relevant to the research) was reviewed; The date of the review; and An assessment of the information reviewed. Subject Materials: Submit any materials that will be provided to, seen or heard by subjects (e.g. questionnaires, scripts, instruction sheet, etc.) Version Date: 05/15/2017 Page 1 of 7 Recruitment Materials: Submit all advertisements and recruitment
material, such as flyers, recruitment e-mails or letters, newspaper advertisements, radio advertisements, social media postings, etc. Tufts MC Clinical Research Recruitment Website form: Submit for all
Tufts MC studies that utilize an Informed Consent Form. This is a Tufts MC institutional requirement and must be submitted in order to receive IRB approval. The form for posting studies on this recruitment website is optional for Tufts University studies. Research COI form: Submit the completed PI’s research COI form as
well as COI forms from research team members who have indicated a “YES” response in any field on the form.
Form 5: Continuing Review
Submit this completed typed form (with original PI signature) to the IRB office (Box 817 or 15 Kneeland St, 1st floor).
On your cover letter, provide a list of each document being submitted for review and approval, categorizing each as follows: New Documents Revised Previously Approved Documents Unchanged Previously Approved Documents The name and version of each listed document should match the name and version on the document itself.
If amendments are being requested, use the Amendment Cover Letter Template as your cover letter for this continuing review. IRB Number: Study Title: PI Telephone: Principal Investigator: PI Email: Research Coordinator Telephone: Research Coordinator: Research Coordinator Email: Institution: Department/Division: Mailing Address or Box #: A. Current Study Status This refers to the status of the protocol under the supervision of the investigator, not the status of the protocol at all centers. If this will be the last continuing review, complete the entirety of the form. Check all that are applicable. 1. The study is permanently closed to enrollment at this site (specify below): Specify date study closed to enrollment: a. Study remains active for Study Intervention and/ or Active Follow-Up consisting of the following activities:
b. All subjects have completed all research-related interventions, and study remains active only for Long-Term Follow-
Up* c. Remaining study activity is limited to data analysis only
*Long-Term Follow-Up includes research interactions that involve no more than minimal risk to subjects (e.g., quality of life surveys); or collection of follow-up data from procedures or interventions that would have been done as part of routine clinical practice to monitor a subject for disease progression or recurrence, regardless of whether the procedures or interventions are described in the research protocol. Long-Term Follow-Up does NOT include research interventions that would not have been performed for clinical purposes, even if the research interventions involve no more than minimal risk. 2. All study related interventions and interactions with subjects have been completed at this site, including interventions and interactions related to collection of long-term follow-up data.
Version Date: 05/15/2017 Page 2 of 7 3. No additional identifiable private information* about the subjects is being obtained by this site’s PI or research team. 4. Analysis of identifiable private information* at this site has been completed. (This can be checked even if a statistical center at another institution will analyze identifiable private information from subjects enrolled at this site.) *Private information is individually identifiable when the identity of the subject is or may readily be ascertained by the investigator or associated with the information. Obtaining identifiable private information includes an investigator’s use, study, or analysis of identifiable private information. With respect to obtaining identifiable private information, this includes obtaining identifiable biological specimens from living individuals. a. The remaining study activity is limited to simply maintaining individually identifiable private information without using,
studying, or analyzing such information. b. The remaining study activity involves only the analysis of aggregate data sets without individual subject identifiers.
If 1c, 2, 3, and 4 (a or b) are all checked, then this is the last continuing review of this study, and the study will be closed with the IRB (proceed to section B. Enrollment Status and continue completing the rest of the form).
If, based on the information you provide, this will be the last continuing review of this study, but you would still like to keep the study active with the IRB, contact the IRB Office Staff to discuss.
If neither 4a nor 4b are checked, continuing review will still be required on an annual basis. 5. Open to enrollment 6. This is a General Certification for a grant that might involve multiple projects. Submit a list of all studies funded by the grant; include: PI name, IRB number, and the last IRB approval date for each study. Include a copy of the grant progress report, if available. 7. Medical record/chart review. Enter the number of medical records/charts reviewed to date: 8. Sample or secondary analysis research only (This does not apply to studies that have closed to enrollment and are conducting data/sample analysis only.)
Version Date: 05/15/2017 Page 3 of 7 B. Enrollment Status In the past year Specify number of: (since the most The IRB counts every subject who signs any informed Complete each field; do not leave fields Total recent continuing consent form (ICF) as enrolled in the study. Potential blank. Indicate 0 or N/A as applicable. review or initial subjects who are screened to determine eligibility are review) counted as enrolled if and only if they sign a screening ICF. 1. Subjects enrolled at this investigator’s site since study started: 2. Screen Failures: *A subject is considered to have withdrawn from the research when the subject either stopped participation or 3. Withdrawals/Discontinuations*: the research team stopped the subject’s participation 4. Subjects who completed the early for reasons other than reaching a study endpoint study: (including subject deaths). Screen failures should not be 5. Subjects remaining in study: counted as withdrawals. If you would like to request an increase in subject enrollment, submit the Amendment Cover Letter Template with this request as your cover letter for this continuing review C. Current Funding Check all that apply 1 Federal Secured Pending Agency Name:
2 Industry Secured Pending Company Name:
Foundation Secured Pending Foundation Name:
Institutional Secured Pending Describe:
Departmental Secured Pending Describe:
Other Secured Pending Describe:
None
Grant title: Specify the primary award recipient of the grant: This is a federal award where Tufts MC/TUHS is receiving direct federal funding and a 310 form is required1
This is a sub-award/contract or service agreement where Tufts MC/TUHS will receive grant funds from the above institution2, 3
This study is funded by another Tufts Medical Center/TUHS IRB approved study (sub-study or ancillary study).
Specify IRB #:
1 If Tufts is the grant recipient, attach Funding/Grant Progress Report. If the most recent progress report is not available, please submit a copy of the grant. documentation that concerns this study (i.e. the research methods and procedures of this study as described in the grant) 2 Industry funding requires a contract is executed with the Tufts Medical Center Clinical Trials Office or the Tufts Office for Technology and Industry Collaboration prior to the commencement of an IRB approved research study. 3 Sub-contract may also include sub recipient, cooperating institution, and other terms sub-award. D. Location of Research Study 1. Specify and describe the location of the research procedures: 2. Other Institutions (specify institutions & attach a copy of each current IRB approval): List other institutions only when Tufts is the Sponsor, primary grant recipient, or coordinating site, otherwise indicate not applicable “N/A”. 3. International Sites (specify sites & attach a copy of each current IRB approval): List international sites only when Tufts is engaged in research at those sites OR is the Sponsor, primary grant recipient, or coordinating site, otherwise indicate not applicable “N/A”. 4. Outside Institutions where Tufts Health Sciences IRB is the designated IRB of record (specify institutions): E. Study-Specific Disclosure of Financial Interest†
Version Date: 05/15/2017 Page 4 of 7 Conflict of Interest (COI) Check to confirm the PI and each research team member have completed the Research COI form and copies of the completed
forms have been retained in the study files.
Check to confirm that the following COI forms are included with this continuing review submission:
1. The PI’s completed COI form (this needs to be submitted regardless of the responses on the form). 2. COI forms from research team members who have indicated a “YES” response in any field on the form.
† Contact the Tufts MC Office of the Vice President of Research or the TUHS Office of the Vice Provost for Research with questions about this COI policy. F. Blood-borne Pathogen Training Annual training is required by the Occupational Safety and Health Administration (OSHA) and the International Air Transit Association (IATA) for any research team member who will collect, handle, and/or process human samples capable of transmitting blood borne pathogens. This does not apply to this research.
This does apply to this research and all research personnel who may come in contact with blood borne pathogens have
completed or will complete the required training and, as applicable, annual updates before conducting research.
Documentation attesting to completed training is subject to audit. Contact the Tufts MC/TUHS Biosafety Officer at (617) 636-0964 with any BBP training related questions.
G. Amendment Provide a summary of any amendments to the research approved and/or pending approval by the IRB since the IRB’s initial review or the most recent continuing review: There have been NO amendments to the research since the IRB’s initial review or the last continuing review. For NIH funded research, please note on 30 July 2015, the NIH released the Guidance on Changes That Involve Human Subjects in Active Awards and That Will Require Prior NIH Approval. Per this guidance, if a study is funded by the NIH, the PI must ensure that any change in research procedures in an active award that would result in an increased risk to human subjects receives NIH approval before implementation. Study modifications are being submitted with this continuing review. If so, use the Amendment Cover Letter Template as your cover letter for this continuing review to describe modifications and provide additional necessary information. Revisions have been made to documents submitted with this continuing review. Submit a list describing which documents have been revised, which are newly submitted, and which remain unchanged. H. Mandatory Human Subjects Protection Education PLEASE NOTE: If additional spaces are needed in this section, please attach an additional piece of paper Current GCP 1 List the PI and Research Training : Individual’s Role Current CITI Team Members This includes Institutional Indicate the expiration (For example, list PI, Co-I, Certification: those responsible for the Affiliation date of each Research Coordinator. Do Indicate the design, conduct, or reporting of (For example, individual’s GCP not list any research team expiration the research, such as the PI & Tufts MC, Training member as a “Co-PI;” only date of each Co-Is, research nurses and TUSM, HNRCA, one person may be listed individual’s CITI coordinators, project etc.) Check N/A if this does as PI for a study.) certification managers, etc. not apply to this study: N/A
1 GCP training is required for NIH funded clinical trials (NIH funded studies that meet the NIH definition of clinical trial). As of 01 January 2018, GCP training will be required for all clinical trial s, regardless of funding source. Does the above list of research team members reflect any changes being made to the research team with this submission? i.e., does section G. Amendment include changes that have not already been reviewed and approved or acknowledged by the IRB? Yes, please acknowledge changes to the research team in the IRB approval letter
Version Date: 05/15/2017 Page 5 of 7 No changes have been made to the research team with this submission
I. Enrollment of Non-English Speaking Subjects
In the past year, have any non-English speaking subjects* been enrolled in this research study?
No - Check all that apply, and provide additional information in the “Other” space, as needed:
Potential non-English speaking subjects did not meet eligibility criteria
Currently, there are no subjects enrolled in the study at all
The study has been closed to subject enrollment for the past year
The IRB approved the exclusion of Non-English speakers in this study
This is a medical record / chart review / secondary analysis
Other:
Yes - Specify which document was used (IRB approved translated ICF or IRB approved short form†):
IRB approved translated ICF; complete table below:
(If re-approval is required, re-submit translated documents for IRB approval and validation) Language
IRB approved short form; complete table below:
Language Number of Times Used
Neither: Check here if a subject (or more than one subjects) was consented without using either 1) an IRB
approved fully translated ICF or 2) an IRB approved short form. If so, this is considered non-compliance: Provide information about the consent process used, and submit a corrective and preventative action plan for enrolling non-English speakers:
Refer to the Tufts Health Sciences IRB Short Form policy or additional information about enrolling non-English speakers.
* Exclusion of non-English speaking subjects from research requires ethical and scientific justification. This justification must be stated in the protocol or Site-Specific Appendix. † If it is expected that more than five (5) persons of a specific non-English speaking population will be enrolled in a twelve (12) month period, the approved English ICF must be translated into the specific foreign language (please refer to the Tufts Health Sciences IRB Translation Policy).
TRUE N/A J. Study activity since the study’s most recent IRB Review (initial or continuing): If Tufts is the Sponsor, primary grant recipient, or coordinating center, complete the below for all participating study sites. Check all that are TRUE or N/A 1. NO subjects have experienced unexpected harm. 2. Anticipated adverse events have NOT taken place with greater frequency or severity than expected or as documented in the protocol, informed consent form, and investigator’s brochure. 3. NO subjects have self-withdrawn from the study.
Version Date: 05/15/2017 Page 6 of 7 4. NO subjects have been withdrawn from the study by the sponsor or PI.
5. There have been NO unanticipated problems*.
6. There have been NO complaints about the study. 7. There has been NO new and relevant information, published or unpublished, about risks associated with the research that the PI is aware of. 8. There have been NO interim findings.
9. There have been NO relevant multi-center trial reports.
10. There have been NO modifications that have not already been submitted to and approved by the IRB.
11. There have been NO regulatory inspections by the FDA or OHRP.
12. There has been NO other relevant information regarding this study, such as information about risks.
13. In the opinion of the Principal Investigator, the risks and potential benefits are unchanged.
14. All problems that require prompt reporting to the IRB have been submitted. 15. The most recent data safety monitoring report is included with this submission in accordance with the timeline specified in the DSMB/DMC Charter. 16. The Data and Safety Monitoring Plan has been implemented and is working as intended.
17. There has been no reportable new information that has not been reported to the IRB. 18. Documents (electronic and/or paper, including executed ICFs, as applicable) are securely retained in a manner that protects privacy and confidentiality of subjects. They are stored in a manner consistent with the plan described in approved study materials (the protocol, Site-Specific Appendix, and ICF). 19. Documentation of current CITI completion is on file for all research team members. For each unchecked statement, include an explanation in the box below: Refer to the item number when providing a response. Include the date of event and subject ID as needed. Attach supporting documents, as needed. If Tufts is the Sponsor, primary grant recipient, or coordinating center, complete the below for all participating study sites, specifying which site each unchecked statement applies to. If the description below includes information that requires prompt reporting, complete a Reportable New Information Form.
*Unanticipated problems are events that meet all 3 of the following criteria: Unexpected (in terms of nature, severity, or frequency) given (a) the research procedures that are described in the protocol- related documents, such as the IRB-approved research protocol and informed consent document; and (b) the characteristics of the subject population being studied; Related or possibly related to participation in the research (in this guidance document, possibly related means there is a reasonable possibility that the incident, experience, or outcome may have been caused by the procedures involved in the research); and Suggests that the research places subjects or others at a greater risk of harm (including physical, psychological, economic, or social harm) than was previously known or recognized. K. Investigator Acknowledgement I understand that I am responsible for protecting the rights, safety, and welfare of each research subject. I will conduct this research in compliance with the protocol, institutional policies including Tufts Health Sciences IRB policies, Investigator Post-Approval Responsibilities and the requirements in the INVESTIGATOR MANUAL (HRP-103), regulations including 45 CFR 46, applicable federal and state laws, and the principles of research ethics set forth in The Belmont Report.
Principal Investigator (type name):
Principal Investigator’s signature: Date:
Version Date: 05/15/2017 Page 7 of 7