Fall 2009 Name: Date:
Experiment 3.7A&B: Solvent and Polarity Effects in Thin-Layer Chromatography
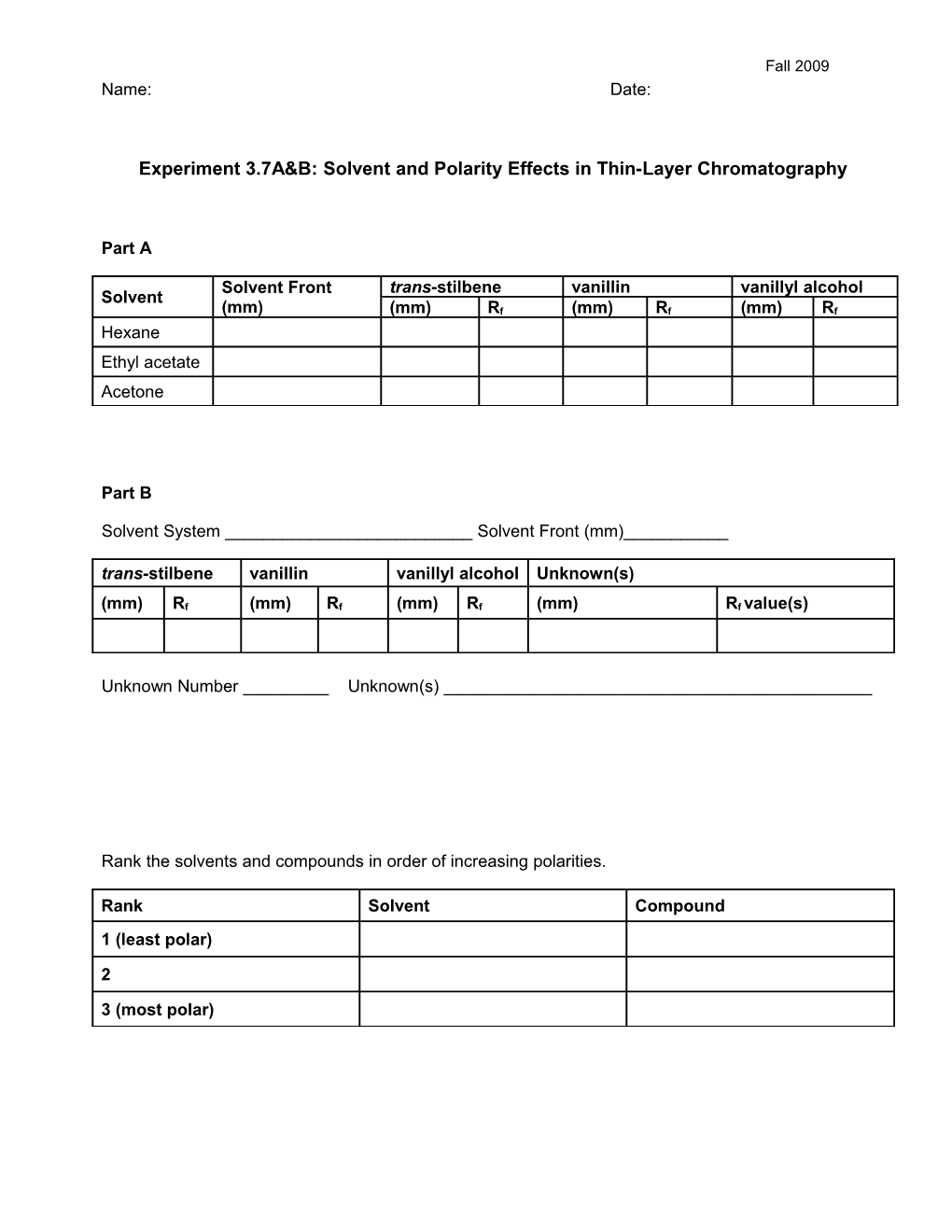
Part A
Solvent Front trans-stilbene vanillin vanillyl alcohol Solvent (mm) (mm) Rf (mm) Rf (mm) Rf Hexane Ethyl acetate Acetone
Part B
Solvent System ______Solvent Front (mm)______trans-stilbene vanillin vanillyl alcohol Unknown(s)
(mm) Rf (mm) Rf (mm) Rf (mm) Rf value(s)
Unknown Number ______Unknown(s) ______
Rank the solvents and compounds in order of increasing polarities.
Rank Solvent Compound 1 (least polar)
2
3 (most polar) Fall 2009
A solvent that gives a high Rf value is said to have a high eluting ability. Rank the solvents in terms of eluting ability for each of the three compounds.
Rank Trans-stilbene Vanillin Vanillyl alcohol 1 (lowest) 2 3 (highest)
1. Do polar or non-polar solvents have greater eluting ability? Explain using observations above.
For each of the solvents used, rank the compounds (trans-stilbene, vanillin, vanillyl alcohol) in terms of
increasing elution rate (increasing Rf value).
Compound with Compound with Compound with Solvent lowest Rf intermediate Rf highest Rf Hexane Ethyl Acetate Acetone
2. Does the order of elution vary according to solvent polarity? Explain using observations above.
3. Do polar or non-polar analytes travel faster on silica gel? Explain using observations above.