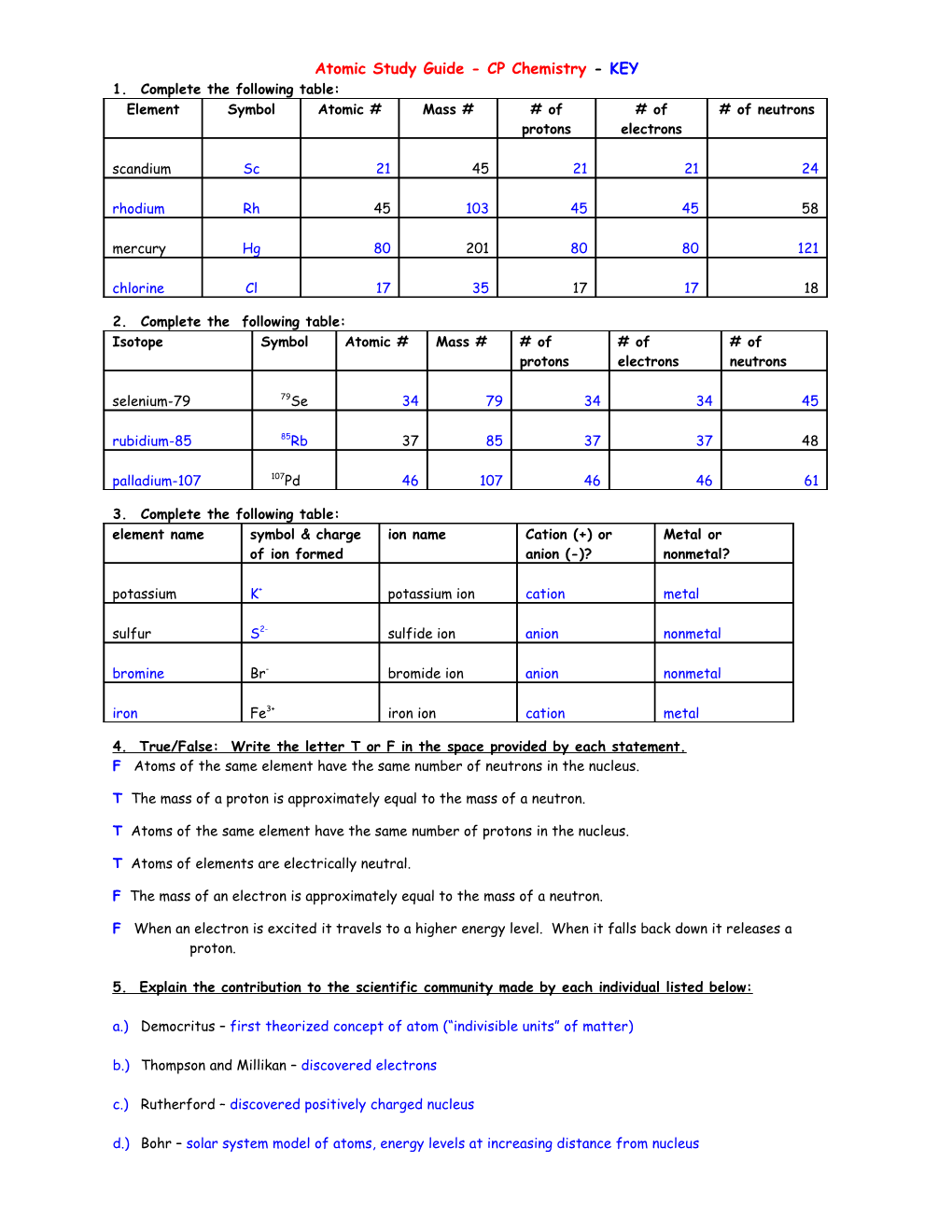
Atomic Study Guide - CP Chemistry - KEY 1. Complete the following table: Element Symbol Atomic # Mass # # of # of # of neutrons protons electrons scandium Sc 21 45 21 21 24 rhodium Rh 45 103 45 45 58 mercury Hg 80 201 80 80 121 chlorine Cl 17 35 17 17 18
2. Complete the following table: Isotope Symbol Atomic # Mass # # of # of # of protons electrons neutrons selenium-79 79Se 34 79 34 34 45 rubidium-85 85Rb 37 85 37 37 48 palladium-107 107Pd 46 107 46 46 61
3. Complete the following table: element name symbol & charge ion name Cation (+) or Metal or of ion formed anion (-)? nonmetal? potassium K+ potassium ion cation metal sulfur S2- sulfide ion anion nonmetal bromine Br- bromide ion anion nonmetal iron Fe3+ iron ion cation metal
4. True/False: Write the letter T or F in the space provided by each statement. F Atoms of the same element have the same number of neutrons in the nucleus.
T The mass of a proton is approximately equal to the mass of a neutron.
T Atoms of the same element have the same number of protons in the nucleus.
T Atoms of elements are electrically neutral.
F The mass of an electron is approximately equal to the mass of a neutron.
F When an electron is excited it travels to a higher energy level. When it falls back down it releases a proton.
5 . Explain the contribution to the scientific community made by each individual listed below: a.) Democritus – first theorized concept of atom (“indivisible units” of matter) b.) Thompson and Millikan – discovered electrons c.) Rutherford – discovered positively charged nucleus d.) Bohr – solar system model of atoms, energy levels at increasing distance from nucleus e.) Mendeleev – first periodic table of elements f.) Moseley – today’s periodic table (arranged by atomic number)
6. Evaluate each part of Dalton’s Atomic Theory listed below. State which points are still completely supported by the scientific community today, and explain why some of the points were disproved or clarified. a.) All elements are made of tiny atoms. Completely supported b.) Atoms cannot be subdivided. Atoms can be subdivided (protons, neutrons, electrons, etc.) but particles do not retain properties of the element c.) Atoms of the same element are exactly alike. Atoms of same element are similar, but can have different numbers of neutrons (isotopes) d.) Atoms of different elements can join to form molecules. Completely supported
7. Write the complete and abbreviated electron configurations for the following atoms and ions: a.) Mg 1s22s22p63s2 [Ne]3s2 b.) Mg2+ 1s22s22p6 [Ne] c.) Mn 1s22s22p63s23p64s23d5 [Ar] 4s23d5
8. Draw the orbital diagram and the Lewis dot diagram for the following elements: a) He He – two dots to the right 1s b) Si _ _ __ Si – four dots – 2 right, 1 up, 1 left 1s 2s 2p 3s 3p c) O _ _ O – 6 dots – 2 right, 2 up, 1 left, 1 down 1s 2s 2p