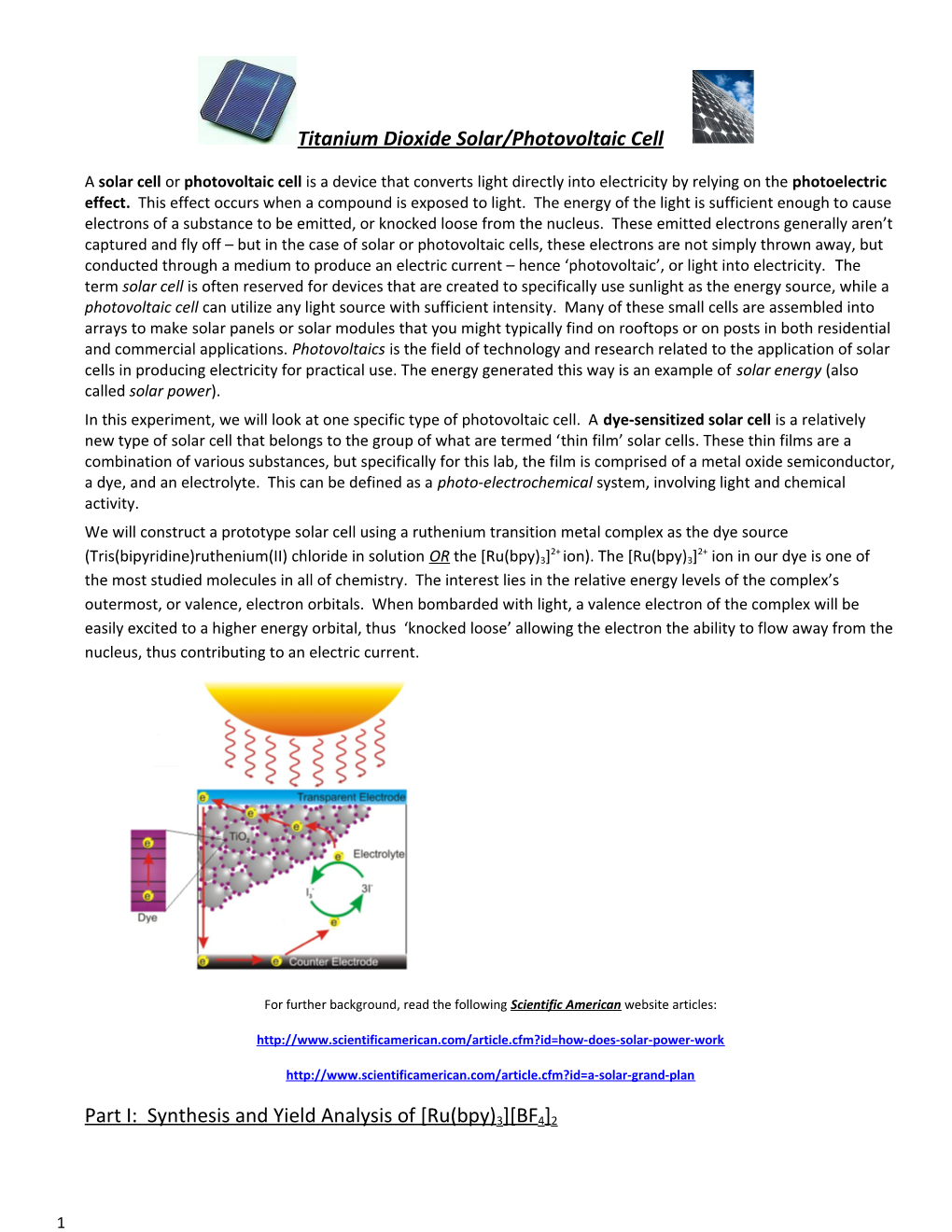
Titanium Dioxide Solar/Photovoltaic Cell
A solar cell or photovoltaic cell is a device that converts light directly into electricity by relying on the photoelectric effect. This effect occurs when a compound is exposed to light. The energy of the light is sufficient enough to cause electrons of a substance to be emitted, or knocked loose from the nucleus. These emitted electrons generally aren’t captured and fly off – but in the case of solar or photovoltaic cells, these electrons are not simply thrown away, but conducted through a medium to produce an electric current – hence ‘photovoltaic’, or light into electricity. The term solar cell is often reserved for devices that are created to specifically use sunlight as the energy source, while a photovoltaic cell can utilize any light source with sufficient intensity. Many of these small cells are assembled into arrays to make solar panels or solar modules that you might typically find on rooftops or on posts in both residential and commercial applications. Photovoltaics is the field of technology and research related to the application of solar cells in producing electricity for practical use. The energy generated this way is an example of solar energy (also called solar power). In this experiment, we will look at one specific type of photovoltaic cell. A dye-sensitized solar cell is a relatively new type of solar cell that belongs to the group of what are termed ‘thin film’ solar cells. These thin films are a combination of various substances, but specifically for this lab, the film is comprised of a metal oxide semiconductor, a dye, and an electrolyte. This can be defined as a photo-electrochemical system, involving light and chemical activity. We will construct a prototype solar cell using a ruthenium transition metal complex as the dye source 2+ 2+ (Tris(bipyridine)ruthenium(II) chloride in solution OR the [Ru(bpy)3] ion). The [Ru(bpy)3] ion in our dye is one of the most studied molecules in all of chemistry. The interest lies in the relative energy levels of the complex’s outermost, or valence, electron orbitals. When bombarded with light, a valence electron of the complex will be easily excited to a higher energy orbital, thus ‘knocked loose’ allowing the electron the ability to flow away from the nucleus, thus contributing to an electric current.
For further background, read the following Scientific American website articles:
http://www.scientificamerican.com/article.cfm?id=how-does-solar-power-work
http://www.scientificamerican.com/article.cfm?id=a-solar-grand-plan
Part I: Synthesis and Yield Analysis of [Ru(bpy)3][BF4]2
1 The dye is synthesized from a ruthenium salt, RuCl3∙3H2O. Pay close attention to the stages of this synthesis. Make note of any observations, i.e. color changes, physical changes of state, etc.
Dissolve 0.083 g RuCl3∙3H2O in 8 mL of water in a 30-mL beaker and add a stir bar.
Add 0.188 g of 2,2’ – dipyridyl and 0.44 mL NaH2PO2. Cover the beaker with a watch glass and reflux for 30 minutes. Refluxing allows liquid in the reaction that has attached to the walls of the container to spill back into the reaction vessel. The purpose is to maintain a constant temperature in the reaction vessel and to prevent solvent from escaping. Why might we want to add heat to this reaction? Add water if necessary during the reflux process to maintain the volume.
To the beaker, add 0.333 g of NaBF4 dissolved in 1.5 mL of water. Let the solution cool to about room temperature and then cool further in ice; crystals should visibly form. Note the color and shape of the crystals in your notes. Collect the product by suction filtration and rinse with cold ethanol (see appendix of lab for details on suction filtration). Air dry and set aside.
Calculate the theoretical yield for this series of reactions. Compare this with the experimental yield by weighing your dried product and calculating percent yield.
Theoretical Yield ______
Experimental Yield ______
Percent Yield = 100∙[experimental yield]/[theoretical yield]
Percent Yield______
Part II: Characterization of [Ru(bpy)3][BF4]2
Characterize your product by UV/visible spectroscopy. Prepare a solution of your product by dissolving 10mg (accurately weighed) in acetonitrile in a 25mL volumetric flask. Perform careful, serial dilutions (see appendix) until you get an absorbance spectrum where the longest wavelength band has an absorbance between 0.5 and 1.0.
Plot absorbance (A) versus the calculated concentration (c) in mol/L and determine the slope of a best-fit line. The slope of this plot is equal to the extinction coefficient (ε) or molar absorptivity coefficient. The extinction coefficient is a proportionality constant that depends on the compound and the wavelength being observed.
It is expressed by Beer’s Law: A = ε l c
where (l) is the path length generally in centimeters. Why do you think the best fit line should begin at the origin of the graph?
Calculate the molar mass of your product (Ru(bpy)3][BF4]2)…(bpy = 2,2’ – bipyridine). Compare this with obtained mass spectrometer results provided.
Calculated Molar Mass ______
Part III: Construction of the thin-fim solar cell
2 Using all the materials noted below, you will now attempt to build a functional thin-film photovoltaic cell that will actually generate a measurable voltage and current.
Diagram of a Completed Solar Cell
Materials for Cell Construction - The following materials will be available:
TiO2 Surfactant (soap) Acetic acid (0.05M) 2 – square cut tin oxide coated glass plates
2 – binder paper clips small mortar and pestle multimeter
Tape glass rod hot plate Petri dish 100mL beaker and ethanol for rinsing
Candle plastic pipets for acetic acid and KI3 light source
You will also need a small amount of your ruthenium product and polyvinyl alcohol (PVA).
Step I: Coating the glass plates with TiO2
1 – Coating glass slides with nano-crystalline Titanium Dioxide: Grind about 0.3 g of titanium dioxide in a mortar and pestle with a few drops of very dilute acetic acid. Alternate grinding with the addition of a few drops of very dilute acetic acid until you obtain a colloidal suspension with a smooth consistency, somewhat like that of latex paint. (Very dilute acetic acid is prepared by adding 0.1 mL concentrated acid to 50 mL of water.) Add a few drops of surfactant or clear dishwashing detergent and mix some more.
2 – Identifying the conductive side of glass: Use a multimeter to measure the resistance of the surfaces of the glass plates (multimeter set to Ohms). The conducting side will have a resistance of 20-30 ohms. Make note of which side is which.
With one plate conducting side up, tape the glass on three edges using one thickness of tape. Wipe off any fingerprints of oils with a tissue wet with ethanol.
3 3 – Coating the glass with Titanium Dioxide: Add some of the titanium dioxide suspension and quickly spread using a glass rod. The tape serves as a 40-50 micrometer spacer to control the thickness of the layer and provides masking for the necessary edge exposure. (If the layer dries out too quickly, add more water.) The layer should be uniformly smooth and little to no air bubbles
should be present. Once applied appropriately and partially dried, carefully remove the tape without scratching the TiO2 coating.
Heat the glass on a hot plate set to HIGH in a hood for 10-15 minutes. The surface initially turns brown as the organic solvent and surfactant (soap) dries and burns off to produce a white and greenish/purple titanium dioxide coating. Reminder** the plate will be hot so allow to cool slowly by simply turning off the hot plate. The coating can easily be chipped off so be careful when moving the plate.
Step II: Preparing the Cell by staining with Ruthenium complex solution and coating the Counter Electrode
1 - Stain the Titanium Dioxide with the Ruthenium Complex: Stain the white side of a glass plate which has been coated with
titanium dioxide (TiO2). This glass has been previously coated with a transparent conductive layer (SnO2), as well as a porous
TiO2 film. Dissolve a very small amount (approximately 0.035 g) of the ruthenium complex in 3-mL of PVA (polyvinyl alcohol) in a
Petri dish. Place the glass slide coated side down in the solution and let stand for 5 minutes. If both sides of the TiO2 film are not uniformly stained, then put it back in the solution for 5 more minutes. Wash the film in ethanol and gently blot it dry with a tissue.
2 - Coat the Counter Electrode: The solar cell needs both a positive and a negative plate to function. The positive electrode is
called the counter electrode and is created from a "conductive" SnO2 coated glass plate. A multimeter can again be used to check which side of the glass is conductive. Pass the second piece of glass, conducting side down, through a candle flame to coat the underside with carbon. For best results, pass the glass quickly and repeatedly through the middle part of the flame.
Wipe off the carbon along the perimeter of three sides of the carbon-coated glass plate using a cotton swab. This will expose edges for a contact point.
4 3 & 4 – Add Electrolyte and Assemble the Finished Solar Cell: The Iodide solution serves as the electrolyte in the solar cell to
complete the circuit and regenerate the ruthenium solution. Add a few drops of a triiodide solution to the TiO2 coated face of
the plate. Add in tiny drops over the full surface to allow the KI3 solution to sufficiently and evenly absorb into the coating. (The
KI3 electrolyte solution consists of 0.5 M KI and 0.05 M I2 in water with about 20% added glycerol.)
Assemble the two glass plates with coated sides together, but offset so that uncoated glass extends beyond the sandwich. Do not rub or slide the plates - simply place and clamp together carefully with binder paper clips. The offsets will serve as the contact points for measuring the voltage.
Step III: Generating and testing for Voltage with the Solar Cell
Connect a Vernier voltage probe or other measuring device (a multimeter can be used as well) by connecting an
alligator clip to each plate (the negative electrode is the TiO2 coated glass and the positive electrode is the carbon coated glass). The Vernier probes are connected to a serial port input on the computer. Click on the desktop icon called ‘Logger Pro’ and follow the instructions provided for using the voltage probe.
Test the current and voltage produced by solar illumination, or... test the current and voltage produced by illumination from an overhead projector as shown above. Do not hold cell too close to overhead beam…place 4-5 inches away. The output should be approximately 0.4 – 0.5 V and 1 mA/cm2 when the cell is illuminated in full sun through the
TiO2 side.
Step IV: Connecting cells in series
Connect multiple solar cells by clipping the negative electrode (TiO2) end of one cell to the positive electrode (carbon-coated) end of the other cell with alligator clips. Connect two remaining ends to the Vernier probe clips as you did with a single cell. Measure the collective voltage generated by two or more cells. What voltage would you expect to generate by doing this?
______
Conclusion
What are some practical uses of thin film solar cells?
5 What purpose did the ruthenium dye serve in the construction of this cell?
What variables might account for the fluctuations in the voltages generated in the same cell and from one cell to the other?
Comment about the pros and cons of solar technology from your observations and reading.
Appendix
Suction Filtration is a method of separation. By using air pressure differences, a greater rate of filtration can be achieved. Gravity provides the force which draws the liquid through the filter paper in standard filtration, but with suction filtration a pressure gradient combined with gravity perform this function. Care must be taken not to use such a strong vacuum as to tear the filter paper or in extreme cases break the Büchner flask.
A serial dilution is the stepwise dilution of a substance in solution. The steps are determined by the user depending on the needs of the experiment. One possibility is a logarithmic dilution that would consist of concentrations decreasing by a power of ten, e.g. from 1.0M, 0.1M, 0.01M, etc. Serial dilutions allow for easier creation of calibration curves and save chemical supplies by relying on one stock source being diluted repeatedly.
6