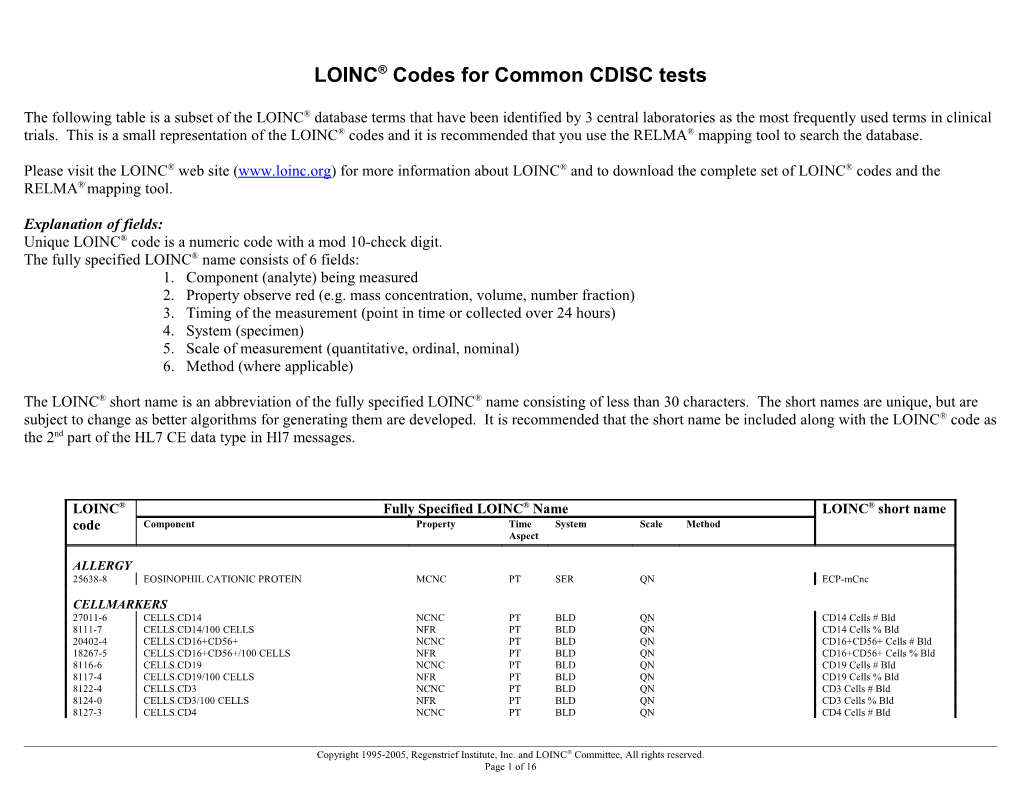
LOINC® Codes for Common CDISC tests
The following table is a subset of the LOINC® database terms that have been identified by 3 central laboratories as the most frequently used terms in clinical trials. This is a small representation of the LOINC® codes and it is recommended that you use the RELMA® mapping tool to search the database.
Please visit the LOINC® web site (www.loinc.org) for more information about LOINC® and to download the complete set of LOINC® codes and the RELMA® mapping tool.
Explanation of fields: Unique LOINC® code is a numeric code with a mod 10-check digit. The fully specified LOINC® name consists of 6 fields: 1. Component (analyte) being measured 2. Property observe red (e.g. mass concentration, volume, number fraction) 3. Timing of the measurement (point in time or collected over 24 hours) 4. System (specimen) 5. Scale of measurement (quantitative, ordinal, nominal) 6. Method (where applicable)
The LOINC® short name is an abbreviation of the fully specified LOINC® name consisting of less than 30 characters. The short names are unique, but are subject to change as better algorithms for generating them are developed. It is recommended that the short name be included along with the LOINC® code as the 2nd part of the HL7 CE data type in Hl7 messages.
LOINC® Fully Specified LOINC® Name LOINC® short name code Component Property Time System Scale Method Aspect
ALLERGY 25638-8 EOSINOPHIL CATIONIC PROTEIN MCNC PT SER QN ECP-mCnc
CELLMARKERS 27011-6 CELLS.CD14 NCNC PT BLD QN CD14 Cells # Bld 8111-7 CELLS.CD14/100 CELLS NFR PT BLD QN CD14 Cells % Bld 20402-4 CELLS.CD16+CD56+ NCNC PT BLD QN CD16+CD56+ Cells # Bld 18267-5 CELLS.CD16+CD56+/100 CELLS NFR PT BLD QN CD16+CD56+ Cells % Bld 8116-6 CELLS.CD19 NCNC PT BLD QN CD19 Cells # Bld 8117-4 CELLS.CD19/100 CELLS NFR PT BLD QN CD19 Cells % Bld 8122-4 CELLS.CD3 NCNC PT BLD QN CD3 Cells # Bld 8124-0 CELLS.CD3/100 CELLS NFR PT BLD QN CD3 Cells % Bld 8127-3 CELLS.CD4 NCNC PT BLD QN CD4 Cells # Bld
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 1 of 16 LOINC® Fully Specified LOINC® Name LOINC® short name code Component Property Time System Scale Method Aspect 8128-1 CELLS.CD4/100 CELLS NFR PT BLD QN CD4 Cells % Bld 8129-9 CELLS.CD4/CELLS.CD8 NRTO PT BLD QN CD4 Cells/CD8 Cells Bld 26982-9 CELLS.CD4+CD25+ NCNC PT BLD QN CD4+CD25+ Cells # Bld 13332-2 CELLS.CD4+CD25+/100 CELLS NFR PT BLD QN CD4+CD25+ Cells % Bld 8137-2 CELLS.CD8 NCNC PT BLD QN CD8 Cells # Bld 8138-0 CELLS.CD8/100 CELLS NFR PT BLD QN CD8 Cells % Bld 18349-1 CELLS.CD8+CD25+ NCNC PT BLD QN CD8+CD25+ Cells # Bld 13334-8 CELLS.CD8+CD25+/100 CELLS NFR PT BLD QN CD8+CD25+ Cells % Bld
CHEMISTRY CHALLENGES 14771-0 GLUCOSE^POST CFST SCNC PT SER/PLAS QN Glucose p fast SerPl-sCnc 1558-6 GLUCOSE^POST CFST MCNC PT SER/PLAS QN Glucose p fast SerPl-mCnc 27873-9 INSULIN^POST CFST ACNC PT SER/PLAS QN Insulin p fast SerPl-aCnc
CHEMISTRY 1690-7 5'-NUCLEOTIDASE CCNC PT SER QN 5NT Ser-cCnc 1715-2 ACID PHOSPHATASE CCNC PT SER/PLAS QN ACP SerPl-cCnc 12173-1 ACID PHOSPHATASE.NON-PROSTATIC CCNC PT SER QN ACP Non-prost Ser-cCnc 1742-6 ALANINE AMINOTRANSFERASE CCNC PT SER/PLAS QN ALT SerPl-cCnc 1751-7 ALBUMIN MCNC PT SER/PLAS QN Alb SerPl-mCnc 1753-3 ALBUMIN ACNC PT UR ORD Alb Ur Ql 14956-7 ALBUMIN MRAT 24H UR QN DETECTION LIMIT <= 20 Microalbumin 24H rate Ur MG/L 14957-5 ALBUMIN MCNC PT UR QN DETECTION LIMIT <= 20 Microalbumin Ur Qn MG/L 30003-8 ALBUMIN MCNC 24H UR QN DETECTION LIMIT <= 20 Microalbumin 24H Cnc Ur MG/L 2862-1 ALBUMIN MCNC PT SER/PLAS QN ELECTROPHORESIS Alb SerPl Elph-mCnc 14959-1 ALBUMIN/CREATININE MCRTO PT UR QN DETECTION LIMIT <= 20 Microalbumin/creat Ur-mRto MG/L 1759-0 ALBUMIN/GLOBULIN MCRTO PT SER/PLAS QN A/G SerPl-mRto 13980-8 ALBUMIN/PROTEIN.TOTAL MFR PT SER/PLAS QN ELECTROPHORESIS Alb % SerPl Elph 13992-3 ALBUMIN/PROTEIN.TOTAL MFR PT UR QN ELECTROPHORESIS Alb % Ur Elph 1761-6 ALDOLASE CCNC PT SER/PLAS QN Aldolase SerPl-cCnc 14586-2 ALDOSTERONE SCNC PT SER/PLAS QN Aldost SerPl-sCnc 15010-2 ALDOSTERONE SCNC PT UR QN Aldost Ur-sCnc 1763-2 ALDOSTERONE MCNC PT SER/PLAS QN Aldost SerPl-mCnc 1764-0 ALDOSTERONE MCNC PT UR QN Aldost Ur-mCnc 15011-0 ALDOSTERONE^SUPINE SCNC PT SER/PLAS QN Aldost sup SerPl-sCnc 1767-3 ALDOSTERONE^SUPINE MCNC PT SER/PLAS QN Aldost sup SerPl-mCnc 15012-8 ALDOSTERONE^UPRIGHT SCNC PT SER/PLAS QN Aldost upr SerPl-sCnc 1768-1 ALDOSTERONE^UPRIGHT MCNC PT SER/PLAS QN Aldost upr SerPl-mCnc 6768-6 ALKALINE PHOSPHATASE CCNC PT SER/PLAS QN ALP SerPl-cCnc 1777-2 ALKALINE PHOSPHATASE.BONE CCNC PT SER/PLAS QN ALP Bone SerPl-cCnc 15013-6 ALKALINE PHOSPHATASE.BONE/ALKALINE CFR PT SER/PLAS QN ALP Bone % SerPl PHOSPHATASE.TOTAL 1778-0 ALKALINE PHOSPHATASE.INTESTINAL CCNC PT SER/PLAS QN ALP Intest SerPl-cCnc 15014-4 ALKALINE PHOSPHATASE.INTESTINAL/ALKALINE CFR PT SER/PLAS QN ALP Intest % SerPl PHOSPHATASE.TOTAL 1779-8 ALKALINE PHOSPHATASE.LIVER CCNC PT SER/PLAS QN ALP Liver SerPl-cCnc 15015-1 ALKALINE PHOSPHATASE.LIVER/ALKALINE CFR PT SER/PLAS QN ALP Liver % SerPl PHOSPHATASE.TOTAL
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 2 of 16 LOINC® Fully Specified LOINC® Name LOINC® short name code Component Property Time System Scale Method Aspect 1825-9 ALPHA 1 ANTITRYPSIN MCNC PT SER/PLAS QN A1AT SerPl-mCnc 2865-4 ALPHA 1 GLOBULIN MCNC PT SER/PLAS QN ELECTROPHORESIS A1 Globulin SerPl Elph- mCnc 13978-2 ALPHA 1 GLOBULIN/PROTEIN.TOTAL MFR PT SER/PLAS QN ELECTROPHORESIS A1 Globulin % SerPl Elph 13990-7 ALPHA 1 GLOBULIN/PROTEIN.TOTAL MFR PT UR QN ELECTROPHORESIS A1 Globulin % Ur Elph 2868-8 ALPHA 2 GLOBULIN MCNC PT SER/PLAS QN ELECTROPHORESIS A2 Globulin SerPl Elph- mCnc 13981-6 ALPHA 2 GLOBULIN/PROTEIN.TOTAL MFR PT SER/PLAS QN ELECTROPHORESIS A2 Globulin % SerPl Elph 13993-1 ALPHA 2 GLOBULIN/PROTEIN.TOTAL MFR PT UR QN ELECTROPHORESIS A2 Globulin % Ur Elph 14590-4 ALPHA TOCOPHEROL SCNC PT SER QN A-Tocopherol Vit E Ser-sCnc 2685-6 ALPHA-1-ACID GLYCOPROTEIN MCNC PT SER/PLAS QN A1Glycoprot SerPl-mCnc 1834-1 ALPHA-1-FETOPROTEIN MCNC PT SER QN AFP Ser-mCnc 2644-3 ALPHA-N-ACETYLGLUCOSAMINIDASE CCNC PT UR QN A-NAG Ur-cCnc 16362-6 AMMONIA SCNC PT PLAS QN Ammonia Plas-sCnc 22763-7 AMMONIA MCNC PT PLAS QN Ammonia Plas-mCnc 1798-8 AMYLASE CCNC PT SER/PLAS QN Amylase SerPl-cCnc 1805-1 AMYLASE.PANCREATIC CCNC PT SER/PLAS QN Amylase P SerPl-cCnc 15057-3 ANDROSTANOLONE SCNC PT SER/PLAS QN Androstanolone SerPl-sCnc 1848-1 ANDROSTANOLONE MCNC PT SER/PLAS QN Androstanolone SerPl-mCnc 14603-5 ANDROSTENEDIONE SCNC PT SER/PLAS QN Androst SerPl-sCnc 1854-9 ANDROSTENEDIONE MCNC PT SER/PLAS QN Androst SerPl-mCnc 1869-7 APOLIPOPROTEIN A-I MCNC PT SER/PLAS QN Apo A-I SerPl-mCnc 1870-5 APOLIPOPROTEIN A-II MCNC PT SER/PLAS QN Apo A-II SerPl-mCnc 1884-6 APOLIPOPROTEIN B MCNC PT SER/PLAS QN Apo B SerPl-mCnc 1877-0 APOLIPOPROTEIN C-III MCNC PT SER/PLAS QN Apo C-III SerPl-mCnc 1886-1 APOLIPOPROTEIN E MCNC PT SER/PLAS QN Apo E SerPl-mCnc 1920-8 ASPARTATE AMINOTRANSFERASE CCNC PT SER/PLAS QN AST SerPl-cCnc 2871-2 BETA GLOBULIN MCNC PT SER/PLAS QN ELECTROPHORESIS B-Globulin SerPl Elph-mCnc 13982-4 BETA GLOBULIN/PROTEIN.TOTAL MFR PT SER/PLAS QN ELECTROPHORESIS B-Globulin % SerPl Elph 13994-9 BETA GLOBULIN/PROTEIN.TOTAL MFR PT UR QN ELECTROPHORESIS B-Globulin % Ur Elph 29512-1 BETA HYDROXYBUTYRATE MCNC PT SER/PLAS QN B-OH-Butyr SerPl-mCnc 6873-4 BETA HYDROXYBUTYRATE SCNC PT SER/PLAS QN B-OH-Butyr SerPl-sCnc 1951-3 BETA-2-MICROGLOBULIN MCNC PT CSF QN B2 Microglob CSF-mCnc 1952-1 BETA-2-MICROGLOBULIN MCNC PT SER QN B2 Microglob Ser-mCnc 1953-9 BETA-2-MICROGLOBULIN MCNC PT UR QN B2 Microglob Ur-mCnc 1959-6 BICARBONATE SCNC PT BLD QN HCO3 Bld-sCnc 1963-8 BICARBONATE SCNC PT SER QN HCO3 Ser-sCnc 14628-2 BILE ACID SCNC PT SER QN Bile Ac Ser-sCnc 14631-6 BILIRUBIN SCNC PT SER/PLAS QN Bilirub SerPl-sCnc 1974-5 BILIRUBIN MCNC PT FLU QN Bilirub Fld-mCnc 1975-2 BILIRUBIN MCNC PT SER/PLAS QN Bilirub SerPl-mCnc 1977-8 BILIRUBIN ACNC PT UR ORD Bilirub Ur Ql 29767-1 BILIRUBIN SCNC PT FLU QN Bilirub Fld-sCnc 14629-0 BILIRUBIN.GLUCURONIDATED+BILIRUBIN.ALBUMIN SCNC PT SER/PLAS QN Bilirub Direct SerPl-sCnc BOUND 1968-7 BILIRUBIN.GLUCURONIDATED+BILIRUBIN.ALBUMIN MCNC PT SER/PLAS QN Bilirub Direct SerPl-mCnc BOUND 14630-8 BILIRUBIN.NON-GLUCURONIDATED SCNC PT SER/PLAS QN Bilirub Indirect SerPl-sCnc 1971-1 BILIRUBIN.NON-GLUCURONIDATED MCNC PT SER/PLAS QN Bilirub Indirect SerPl-mCnc 14633-2 C PEPTIDE SCNC PT SER/PLAS QN C Peptide SerPl-sCnc 1986-9 C PEPTIDE MCNC PT SER/PLAS QN C Peptide SerPl-mCnc 1988-5 C REACTIVE PROTEIN MCNC PT SER/PLAS QN CRP SerPl-mCnc
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 3 of 16 LOINC® Fully Specified LOINC® Name LOINC® short name code Component Property Time System Scale Method Aspect 30522-7 C REACTIVE PROTEIN MCNC PT SER/PLAS QN HIGH SENSITIVITY CRP SerPl High Sens-mCnc 14635-7 CALCIDIOL SCNC PT SER/PLAS QN Vit D25 SerPl-sCnc 1989-3 CALCIDIOL MCNC PT SER/PLAS QN Vit D25 SerPl-mCnc 14637-3 CALCIUM SRAT 24H UR QN Calcium 24H Ur-sRate 17861-6 CALCIUM MCNC PT SER/PLAS QN Calcium SerPl-mCnc 17862-4 CALCIUM MCNC PT UR QN Calcium Ur-mCnc 18488-7 CALCIUM MCNC 24H UR QN Calcium 24H Ur-mCnc 2000-8 CALCIUM SCNC PT SER/PLAS QN Calcium SerPl-sCnc 2004-0 CALCIUM SCNC PT UR QN Calcium Ur-sCnc 25362-5 CALCIUM SCNC 24H UR QN Calcium 24H Ur-sCnc 1995-0 CALCIUM.IONIZED SCNC PT SER/PLAS QN Ca-I SerPl-sCnc 18281-6 CALCIUM^^CORRECTED FOR TOTAL PROTEIN SCNC PT SER QN Calcium TP cor Ser-sCnc 24108-3 CANCER AG 19-9 ACNC PT SER/PLAS QN Cancer Ag19-9 SerPl-aCnc 2028-9 CARBON DIOXIDE SCNC PT SER/PLAS QN CO2 SerPl-sCnc 20563-3 CARBOXYHEMOGLOBIN/HEMOGLOBIN.TOTAL MFR PT BLD QN COHgb % Bld 2039-6 CARCINOEMBRYONIC AG MCNC PT SER/PLAS QN CEA SerPl-mCnc 14287-7 CARNITINE SCNC PT UR QN Carnitine Ur-sCnc 14288-5 CARNITINE SCNC PT SER/PLAS QN Carnitine SerPl-sCnc 24448-3 CARNITINE SRAT 24H UR QN Carnitine 24H Ur-sRate 14285-1 CARNITINE.FREE SCNC PT UR QN Carnitine Free Ur-sCnc 14286-9 CARNITINE.FREE SCNC PT SER/PLAS QN Carnitine Free SerPl-sCnc 2064-4 CERULOPLASMIN MCNC PT SER QN Ceruloplasmin Ser-mCnc 2075-0 CHLORIDE SCNC PT SER/PLAS QN Chloride SerPl-sCnc 2078-4 CHLORIDE SCNC PT UR QN Chloride Ur-sCnc 21194-6 CHLORIDE SCNC 24H UR QN Chloride 24H Ur-sCnc 14647-2 CHOLESTEROL SCNC PT SER/PLAS QN Cholest SerPl-sCnc 2093-3 CHOLESTEROL MCNC PT SER/PLAS QN Cholest SerPl-mCnc 14646-4 CHOLESTEROL.IN HDL SCNC PT SER/PLAS QN HDLc SerPl-sCnc 2085-9 CHOLESTEROL.IN HDL MCNC PT SER/PLAS QN HDLc SerPl-mCnc 26017-4 CHOLESTEROL.IN HDL 3 SCNC PT SER/PLAS QN HDL3c SerPl-sCnc 9833-5 CHOLESTEROL.IN HDL 3 MCNC PT SER/PLAS QN HDL3c SerPl-mCnc 2089-1 CHOLESTEROL.IN LDL MCNC PT SER/PLAS QN LDLc SerPl-mCnc 22748-8 CHOLESTEROL.IN LDL SCNC PT SER/PLAS QN LDLc SerPl-sCnc 18262-6 CHOLESTEROL.IN LDL MCNC PT SER/PLAS QN DIRECT ASSAY LDLc SerPl Direct Assay- mCnc 2091-7 CHOLESTEROL.IN VLDL MCNC PT SER/PLAS QN VLDLc SerPl-mCnc 25371-6 CHOLESTEROL.IN VLDL SCNC PT SER/PLAS QN VLDLc SerPl-sCnc 2110-5 CHORIOGONADOTROPIN.BETA SUBUNIT ACNC PT SER ORD B-HCG Ser Ql 21198-7 CHORIOGONADOTROPIN.BETA SUBUNIT ACNC PT SER QN B-HCG Ser-aCnc 6687-8 CITRATE MRAT 24H UR QN Citrate 24H Ur-mRate 14685-2 COBALAMINS SCNC PT SER QN Vit B12 Ser-sCnc 2132-9 COBALAMINS MCNC PT SER QN Vit B12 Ser-mCnc 21215-9 COLLAGEN CROSSLINKED N-TELOPEPTIDE SCNC PT SER QN Collagen NTx Ser-sCnc 27939-8 COLLAGEN CROSSLINKED N-TELOPEPTIDE SCNC PT UR QN Collagen NTx Ur-sCnc 14115-0 COLLAGEN CROSSLINKED N- SCRTO PT UR QN Collagen NTx/creat Ur-sRto TELOPEPTIDE/CREATININE 14674-6 CORTICOTROPIN SCNC PT PLAS QN ACTH Plas-sCnc 2141-0 CORTICOTROPIN MCNC PT PLAS QN ACTH Plas-mCnc 14675-3 CORTISOL SCNC PT SER/PLAS QN Cortis SerPl-sCnc 2143-6 CORTISOL MCNC PT SER/PLAS QN Cortis SerPl-mCnc 25883-0 CORTISOL.FREE SCNC 24H UR QN HPLC Cortis Free 24H Ur HPLC- sCnc
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 4 of 16 LOINC® Fully Specified LOINC® Name LOINC® short name code Component Property Time System Scale Method Aspect 14679-5 CORTISOL^MORNING SCNC PT SER/PLAS QN Cortis AM SerPl-sCnc 2157-6 CREATINE KINASE CCNC PT SER/PLAS QN CK SerPl-cCnc 2152-7 CREATINE KINASE.BB CCNC PT SER/PLAS QN ELECTROPHORESIS CK BB SerPl Elph-cCnc 9642-0 CREATINE KINASE.BB/CREATINE KINASE.TOTAL CFR PT SER/PLAS QN CK BB % SerPl 13969-1 CREATINE KINASE.MB MCNC PT SER/PLAS QN CK MB SerPl-mCnc 6773-6 CREATINE KINASE.MB CCNC PT SER/PLAS QN EIA CK MB SerPl EIA-cCnc 2154-3 CREATINE KINASE.MB CCNC PT SER/PLAS QN ELECTROPHORESIS CK MB SerPl Elph-cCnc 20569-0 CREATINE KINASE.MB/CREATINE KINASE.TOTAL CFR PT SER/PLAS QN CK MB % SerPl 2155-0 CREATINE KINASE.MM CCNC PT SER/PLAS QN ELECTROPHORESIS CK MM SerPl Elph-cCnc 9643-8 CREATINE KINASE.MM/CREATINE KINASE.TOTAL CFR PT SER/PLAS QN CK MM % SerPl 14682-9 CREATININE SCNC PT SER/PLAS QN Creat SerPl-sCnc 14683-7 CREATININE SCNC PT UR QN Creat Ur-sCnc 14684-5 CREATININE SRAT 24H UR QN Creat 24H Ur-sRate 15051-6 CREATININE SCNC PT DIAF QN Creat Diaf-sCnc 20624-3 CREATININE MCNC 24H UR QN Creat 24H Ur-mCnc 2160-0 CREATININE MCNC PT SER/PLAS QN Creat SerPl-mCnc 2161-8 CREATININE MCNC PT UR QN Creat Ur-mCnc 2162-6 CREATININE MRAT 24H UR QN Creat 24H Ur-mRate 25886-3 CREATININE SCNC 24H UR QN Creat 24H Ur-sCnc 5919-6 CREATININE MCNC PT DIAF QN Creat Diaf-mCnc 15054-0 DEHYDROEPIANDROSTERONE SCNC PT SER/PLAS QN DHEA SerPl-sCnc 2193-1 DEHYDROEPIANDROSTERONE MCNC PT SER/PLAS QN DHEA SerPl-mCnc 14688-6 DEHYDROEPIANDROSTERONE SULFATE SCNC PT SER/PLAS QN DHEA-S SerPl-sCnc 2191-5 DEHYDROEPIANDROSTERONE SULFATE MCNC PT SER/PLAS QN DHEA-S SerPl-mCnc 27424-1 DEOXYPYRIDINOLINE SCNC PT UR QN DPD Ur-sCnc 25095-1 DEOXYPYRIDINOLINE/CREATININE SCRTO PT UR QN DPD/creat Ur-sRto 15061-5 ERYTHROPOIETIN ACNC PT SER/PLAS QN EPO SerPl-aCnc 14715-7 ESTRADIOL SCNC PT SER/PLAS QN Estradiol SerPl-sCnc 2243-4 ESTRADIOL MCNC PT SER/PLAS QN Estradiol SerPl-mCnc 2254-1 ESTROGEN MCNC PT SER/PLAS QN Estrogen SerPl-mCnc 22663-9 ESTRONE SCNC PT SER/PLAS QN Estrone SerPl-sCnc 15066-4 FATTY ACIDS.NONESTERIFIED SCNC PT SER/PLAS QN NEFA SerPl-sCnc 14723-1 FERRITIN SCNC PT SER QN Ferritin Ser-sCnc 2276-4 FERRITIN MCNC PT SER QN Ferritin Ser-mCnc 14732-2 FOLATE SCNC PT SER QN Folate Ser-sCnc 2284-8 FOLATE MCNC PT SER QN Folate Ser-mCnc 15067-2 FOLLITROPIN ACNC PT SER/PLAS QN FSH SerPl-aCnc 15069-8 FRUCTOSAMINE SCNC PT SER/PLAS QN Fructosamine SerPl-sCnc 2874-6 GAMMA GLOBULIN MCNC PT SER/PLAS QN ELECTROPHORESIS G-Globulin SerPl Elph-mCnc 13983-2 GAMMA GLOBULIN/PROTEIN.TOTAL MFR PT SER/PLAS QN ELECTROPHORESIS G-Globulin % SerPl Elph 13995-6 GAMMA GLOBULIN/PROTEIN.TOTAL MFR PT UR QN ELECTROPHORESIS G-Globulin % Ur Elph 2324-2 GAMMA GLUTAMYL TRANSFERASE CCNC PT SER/PLAS QN GGT SerPl-cCnc 2333-3 GASTRIN MCNC PT SER/PLAS QN Gastrin SerPl-mCnc 2336-6 GLOBULIN MCNC PT SER QN Globulin Ser-mCnc 14749-6 GLUCOSE SCNC PT SER/PLAS QN Glucose SerPl-sCnc 15076-3 GLUCOSE SCNC PT UR QN Glucose Ur-sCnc 2345-7 GLUCOSE MCNC PT SER/PLAS QN Glucose SerPl-mCnc 2349-9 GLUCOSE ACNC PT UR ORD Glucose Ur Ql 2350-7 GLUCOSE MCNC PT UR QN Glucose Ur-mCnc 2415-8 HISTAMINE MCNC PT BLD QN Histamine Bld-mCnc 13965-9 HOMOCYSTEINE SCNC PT SER/PLAS QN Homocysteine SerPl-sCnc 2458-8 IGA MCNC PT SER QN IgA Ser-mCnc
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 5 of 16 LOINC® Fully Specified LOINC® Name LOINC® short name code Component Property Time System Scale Method Aspect 19113-0 IGE ACNC PT SER QN IgE Ser-aCnc 2465-3 IGG MCNC PT SER QN IgG Ser-mCnc 2472-9 IGM MCNC PT SER QN IgM Ser-mCnc 20448-7 INSULIN ACNC PT SER/PLAS QN Insulin SerPl-aCnc 25447-4 INSULIN.FREE SCNC PT SER/PLAS QN Insulin Free SerPl-sCnc 2483-6 INSULIN-LIKE GROWTH FACTOR BINDING PROTEIN MCNC PT SER/PLAS QN IGF BP3 SerPl-mCnc 3 2484-4 INSULIN-LIKE GROWTH FACTOR-I MCNC PT SER/PLAS QN IGF-I SerPl-mCnc 29775-4 INSULIN-LIKE GROWTH FACTOR-I SCNC PT SER/PLAS QN IGF-I SerPl-sCnc 13440-3 INTERPRETATION IMP PT UR NOM IMMUNOFIXATION Interpretation Ur IFE-Imp 14798-3 IRON SCNC PT SER/PLAS QN Iron SerPl-sCnc 2498-4 IRON MCNC PT SER/PLAS QN Iron SerPl-mCnc 25937-4 IRON SCNC 24H UR QN Iron 24H Ur-sCnc 30030-1 IRON MCNC 24H UR QN Iron 24H Ur-mCnc 14800-7 IRON BINDING CAPACITY SCNC PT SER/PLAS QN TIBC SerPl-sCnc 2500-7 IRON BINDING CAPACITY MCNC PT SER/PLAS QN TIBC SerPl-mCnc 22753-8 IRON BINDING CAPACITY.UNSATURATED SCNC PT SER/PLAS QN UIBC SerPl-sCnc 2501-5 IRON BINDING CAPACITY.UNSATURATED MCNC PT SER/PLAS QN UIBC SerPl-mCnc 2514-8 KETONES ACNC PT UR ORD TEST STRIP Ketones Ur Ql Strip 14118-4 LACTATE MCNC PT SER/PLAS QN Lactate SerPl-mCnc 2524-7 LACTATE SCNC PT SER/PLAS QN Lactate SerPl-sCnc 2532-0 LACTATE DEHYDROGENASE CCNC PT SER/PLAS QN LDH SerPl-cCnc 2534-6 LACTATE DEHYDROGENASE CCNC PT UR QN LDH Ur-cCnc 2537-9 LACTATE DEHYDROGENASE 1 CCNC PT SER/PLAS QN LDH1 SerPl-cCnc 2540-3 LACTATE DEHYDROGENASE 2 CCNC PT SER/PLAS QN LDH2 SerPl-cCnc 2543-7 LACTATE DEHYDROGENASE 3 CCNC PT SER/PLAS QN LDH3 SerPl-cCnc 2546-0 LACTATE DEHYDROGENASE 4 CCNC PT SER/PLAS QN LDH4 SerPl-cCnc 2549-4 LACTATE DEHYDROGENASE 5 CCNC PT SER/PLAS QN LDH5 SerPl-cCnc 21365-2 LEPTIN MCNC PT SER/PLAS QN Leptin SerPl-mCnc 10835-7 LIPOPROTEIN (LITTLE A) MCNC PT SER/PLAS QN LPa SerPl-mCnc 10501-5 LUTROPIN ACNC PT SER/PLAS QN LH SerPl-aCnc 19123-9 MAGNESIUM MCNC PT SER/PLAS QN Magnesium SerPl-mCnc 19124-7 MAGNESIUM MCNC PT UR QN Magnesium Ur-mCnc 25954-9 MAGNESIUM SCNC 24H UR QN Magnesium 24H Ur-sCnc 2598-1 MAGNESIUM SCNC PT UR QN Magnesium Ur-sCnc 2601-3 MAGNESIUM SCNC PT SER/PLAS QN Magnesium SerPl-sCnc 2639-3 MYOGLOBIN MCNC PT SER/PLAS QN Myoglobin SerPl-mCnc 2641-9 MYOGLOBIN MCNC PT UR QN Myoglobin Ur-mCnc 30934-4 NATRIURETIC PEPTIDE.B MCNC PT SER/PLAS QN BNP SerPl-mCnc 9740-2 NEOPTERIN SCNC PT SER/PLAS QN Neopterin SerPl-sCnc 2695-5 OSMOLALITY OSMOL PT UR QN Osmolality Ur Qn 2692-2 OSMOLALITY OSMOL PT SER/PLAS QN Osmolality SerPl Qn 2694-8 OSMOLALITY OSMOL 24H UR QN Osmolality 24H Ur Qn 15084-7 OSTEOCALCIN SCNC PT SER QN Osteocalcin Ser-sCnc 2697-1 OSTEOCALCIN MCNC PT SER QN Osteocalcin Ser-mCnc 2713-6 OXYGEN SATURATION.CALC FROM OXYGEN SFR PT BLD QN O2 Satn from pO2 % Bld PARTIAL PRESSURE 14866-8 PARATHYRIN.INTACT SCNC PT SER/PLAS QN PTH-Intact SerPl-sCnc 2731-8 PARATHYRIN.INTACT MCNC PT SER/PLAS QN PTH-Intact SerPl-mCnc 27378-9 PH SCNC 24H UR QN pH 24H Ur-sCnc 2753-2 PH SCNC PT SER/PLAS QN pH SerPl-sCnc 2756-5 PH SCNC PT UR QN pH Ur-sCnc
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 6 of 16 LOINC® Fully Specified LOINC® Name LOINC® short name code Component Property Time System Scale Method Aspect 13539-2 PHOSPHATE SCNC PT UR QN Phosphate Ur-sCnc 14879-1 PHOSPHATE SCNC PT SER/PLAS QN Phosphate SerPl-sCnc 14881-7 PHOSPHATE SRAT 24H UR QN Phosphate 24H Ur-sRate 21458-5 PHOSPHATE MCNC 24H UR QN Phosphate 24H Ur-mCnc 25973-9 PHOSPHATE SCNC 24H UR QN Phosphate 24H Ur-sCnc 2775-5 PHOSPHATE MCNC PT DIAF QN Phosphate Diaf-mCnc 2777-1 PHOSPHATE MCNC PT SER/PLAS QN Phosphate SerPl-mCnc 2778-9 PHOSPHATE MCNC PT UR QN Phosphate Ur-mCnc 2779-7 PHOSPHATE MRAT 24H UR QN Phosphate 24H Ur-mRate 21476-7 POTASSIUM SCNC 24H UR QN Potassium 24H Ur-sCnc 2823-3 POTASSIUM SCNC PT SER/PLAS QN Potassium SerPl-sCnc 2828-2 POTASSIUM SCNC PT UR QN Potassium Ur-sCnc 2829-0 POTASSIUM SRAT 24H UR QN Potassium 24H Ur-sRate 14890-8 PROGESTERONE SCNC PT SER/PLAS QN Progest SerPl-sCnc 2839-9 PROGESTERONE MCNC PT SER/PLAS QN Progest SerPl-mCnc 2842-3 PROLACTIN MCNC PT SER/PLAS QN Prolactin SerPl-mCnc 2857-1 PROSTATE SPECIFIC AG MCNC PT SER/PLAS QN PSA SerPl-mCnc 21482-5 PROTEIN MCNC 24H UR QN Prot 24H Ur-mCnc 2885-2 PROTEIN MCNC PT SER/PLAS QN Prot SerPl-mCnc 2889-4 PROTEIN MRAT 24H UR QN Prot 24H Ur-mRate 2890-2 PROTEIN/CREATININE MCRTO PT UR QN Prot/creat Ur-mRto 25977-0 PYRIDINOLINE SCNC 24H UR QN PYD 24H Ur-sCnc 25129-8 PYRIDINOLINE/CREATININE SCRTO PT UR QN PYD/creat Ur-sRto 13967-5 SEX HORMONE BINDING GLOBULIN SCNC PT SER QN SHBG Ser-sCnc 21525-1 SODIUM SCNC 24H UR QN Sodium 24H Ur-sCnc 2951-2 SODIUM SCNC PT SER/PLAS QN Sodium SerPl-sCnc 2955-3 SODIUM SCNC PT UR QN Sodium Ur-sCnc 2956-1 SODIUM SRAT 24H UR QN Sodium 24H Ur-sRate 16257-8 SODIUM URATE CRYSTALS ACNC PT CALC ORD INFRARED Na Urate Cry Calc Ql IR SPECTROSCOPY 2963-7 SOMATOTROPIN MCNC PT SER/PLAS QN GH SerPl-mCnc 2965-2 SPECIFIC GRAVITY RDEN PT UR QN Sp Gr Ur Qn 14913-8 TESTOSTERONE SCNC PT SER/PLAS QN Testost SerPl-sCnc 2986-8 TESTOSTERONE MCNC PT SER/PLAS QN Testost SerPl-mCnc 14914-6 TESTOSTERONE.FREE SCNC PT SER/PLAS QN Testost Free SerPl-sCnc 2991-8 TESTOSTERONE.FREE MCNC PT SER/PLAS QN Testost Free SerPl-mCnc 11061-9 THROMBOXANE BETA 2 MRAT 24H UR QN TXB2 24H Ur-mRate 3016-3 THYROTROPIN ACNC PT SER/PLAS QN TSH SerPl-aCnc 14921-1 THYROXINE SCNC PT SER/PLAS QN T4 SerPl-sCnc 27980-2 THYROXINE BINDING GLOBULIN SCNC PT SER/PLAS QN T4BG SerPl-sCnc 3021-3 THYROXINE BINDING GLOBULIN MCNC PT SER/PLAS QN T4BG SerPl-mCnc 32215-6 THYROXINE FREE INDEX ACNC PT SER/PLAS QN FTI SerPl-aCnc 14920-3 THYROXINE.FREE SCNC PT SER/PLAS QN Free T4 SerPl-sCnc 3024-7 THYROXINE.FREE MCNC PT SER/PLAS QN Free T4 SerPl-mCnc 22674-6 TRANSFERRIN SCNC PT SER/PLAS QN Transferrin SerPl-sCnc 3034-6 TRANSFERRIN MCNC PT SER/PLAS QN Transferrin SerPl-mCnc 30248-9 TRANSFERRIN RECEPTOR.SOLUBLE MCNC PT SER/PLAS QN sTfR SerPl-mCnc 3040-3 TRIACYLGLYCEROL LIPASE CCNC PT SER/PLAS QN Lipase SerPl-cCnc 14927-8 TRIGLYCERIDE SCNC PT SER/PLAS QN Trigl SerPl-sCnc 2571-8 TRIGLYCERIDE MCNC PT SER/PLAS QN Trigl SerPl-mCnc 14930-2 TRIIODOTHYRONINE SCNC PT SER/PLAS QN T3 SerPl-sCnc 3053-6 TRIIODOTHYRONINE MCNC PT SER/PLAS QN T3 SerPl-mCnc
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 7 of 16 LOINC® Fully Specified LOINC® Name LOINC® short name code Component Property Time System Scale Method Aspect 3050-2 TRIIODOTHYRONINE RESIN UPTAKE NFR PT SER/PLAS QN T3RU % SerPl 14928-6 TRIIODOTHYRONINE.FREE SCNC PT SER/PLAS QN T3Free SerPl-sCnc 3051-0 TRIIODOTHYRONINE.FREE MCNC PT SER/PLAS QN T3Free SerPl-mCnc 14929-4 TRIIODOTHYRONINE.REVERSE SCNC PT SER/PLAS QN T3Reverse SerPl-sCnc 3052-8 TRIIODOTHYRONINE.REVERSE MCNC PT SER/PLAS QN T3Reverse SerPl-mCnc 10839-9 TROPONIN I.CARDIAC MCNC PT SER/PLAS QN Troponin I SerPl-mCnc 6598-7 TROPONIN T.CARDIAC MCNC PT SER/PLAS QN Troponin T SerPl-mCnc 21582-2 TRYPTASE MCNC PT SER/PLAS QN Tryptase SerPl-mCnc 13900-6 TUMOR NECROSIS FACTOR.ALPHA MCNC PT SER QN A-Tumor Necrosis Fact Ser- mCnc 14933-6 URATE SCNC PT SER/PLAS QN Urate SerPl-sCnc 14934-4 URATE SCNC PT UR QN Urate Ur-sCnc 21587-1 URATE MCNC 24H UR QN Urate 24H Ur-mCnc 25997-8 URATE SCNC 24H UR QN Urate 24H Ur-sCnc 3084-1 URATE MCNC PT SER/PLAS QN Urate SerPl-mCnc 3086-6 URATE MCNC PT UR QN Urate Ur-mCnc 22664-7 UREA SCNC PT SER/PLAS QN Urea SerPl-sCnc 14936-9 UREA NITROGEN SCNC PT DIAFP QN Urea Nit DiafP-sCnc 14937-7 UREA NITROGEN SCNC PT SER/PLAS QN BUN SerPl-sCnc 14938-5 UREA NITROGEN SCNC PT UR QN UUN Ur-sCnc 3094-0 UREA NITROGEN MCNC PT SER/PLAS QN BUN SerPl-mCnc 3095-7 UREA NITROGEN MCNC PT UR QN UUN Ur-mCnc 5918-8 UREA NITROGEN MCNC PT DIAF QN Urea Nit Diaf-mCnc 3097-3 UREA NITROGEN/CREATININE MCRTO PT SER/PLAS QN BUN/creat SerPl-mRto 13658-0 UROBILINOGEN ACNC PT UR ORD Urobilinogen Ur Ql 3107-0 UROBILINOGEN MCNC PT UR QN Urobilinogen Ur-mCnc
COAGULATION 27811-9 ANTITHROMBIN ACTUAL/NORMAL RLCCNC PT PPP QN ENZY AT III Act/Nor PPP Chro 27812-7 ANTITHROMBIN AG ACTUAL/NORMAL RLMCNC PT PPP QN IMM AT III Ag Act/Nor PPP Imm 8065-5 CARDIOLIPIN AB.IGG ACNC PT SER QN Cardiolipin IgG Ser-aCnc 8067-1 CARDIOLIPIN AB.IGM ACNC PT SER QN Cardiolipin IgM Ser-aCnc 3218-5 COAGULATION FACTOR X ACTIVITY RLTM PT PPP QN COAG FX Act/Nor PPP Qn ACTUAL/NORMAL 5904-8 COAGULATION RUSSELL VIPER VENOM INDUCED TIME PT PPP QN COAG Coag RVV Ind PPP Qn 13488-2 COAGULATION SURFACE INDUCED TIME PT PPP^CONTROL QN COAG aPTT PPP Cont Qn 14979-9 COAGULATION SURFACE INDUCED TIME PT PPP QN COAG aPTT PPP Qn 3173-2 COAGULATION SURFACE INDUCED TIME PT BLD QN COAG aPTT Bld Qn 5946-9 COAGULATION SURFACE INDUCED.FACTOR TIME PT PPP QN COAG aPTT imm NP PPP Qn SUBSTITUTION^IMMEDIATELY AFTER ADDITION OF NORMAL PLASMA 3243-3 COAGULATION THROMBIN INDUCED TIME PT PPP QN COAG TT PPP Qn 5901-4 COAGULATION TISSUE FACTOR INDUCED TIME PT PPP^CONTROL QN COAG PT PPP Cont Qn 5902-2 COAGULATION TISSUE FACTOR INDUCED TIME PT PPP QN COAG PT PPP Qn 6301-6 COAGULATION TISSUE FACTOR INDUCED.INR RLTM PT PPP QN COAG INR PPP Qn 30240-6 FIBRIN D-DIMER MCNC PT PPP QN D Dimer PPP-mCnc 3255-7 FIBRINOGEN MCNC PT PPP QN COAG Fibrinogen PPP-mCnc 22758-7 PLASMINOGEN ACTIVATOR INHIBITOR 1 AG MCNC PT PPP QN IMM PAI1 Ag PPP Imm-mCnc 24377-4 PLATELET AGGREGATION.ADENOSINE ACNC PT PRP ORD IPA ADP PRP Ql DIPHOSPHATE INDUCED 24379-0 PLATELET AGGREGATION.COLLAGEN INDUCED ACNC PT PRP ORD IPA Coll PRP Ql 14182-0 THROMBIN ANTITHROMBIN COMPLEX AG MCNC PT PPP QN IMM TAT Ag PPP Imm-mCnc
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 8 of 16 LOINC® Fully Specified LOINC® Name LOINC® short name code Component Property Time System Scale Method Aspect 27816-8 VON WILLEBRAND FACTOR AG ACTUAL/NORMAL RLMCNC PT PPP QN IMM vWf Ag Act/Nor PPP Imm
CYTOLOGY 19767-3 CYTOLOGIST ID PT CVX/VAG NOM CYTO STAIN Cytologist Cvx/Vag Cyto 19774-9 CYTOLOGY STUDY COMMENT IMP PT CVX/VAG NAR CYTO STAIN Cytology Cmnt Cvx/Vag Cyto-Imp 19762-4 GENERAL CATEGORIES IMP PT CVX/VAG NOM CYTO STAIN Gen Categ Cvx/Vag Cyto-Imp 19769-9 PATHOLOGIST ID PT CVX/VAG NOM CYTO STAIN Pathologist Cvx/Vag Cyto 19773-1 RECOMMENDED FOLLOWUP PRID PT CVX/VAG NOM CYTO STAIN Recom F/U Cvx/Vag Cyto 19763-2 SPECIMEN SOURCE PRID PT CVX/VAG NOM CYTO STAIN Spec Source Cvx/Vag Cyto 19764-0 STATEMENT OF ADEQUACY IMP PT CVX/VAG NOM CYTO STAIN Stat of Adq Cvx/Vag Cyto- Imp
DRUG LEVELS & TOXICOLOGY 8150-5 AMPHETAMINES MCNC PT UR QN Amphets Ur-mCnc 16369-1 AMPHETAMINES ACNC PT UR ORD CONFIRM Amphets Ur Ql Cfm 19261-7 AMPHETAMINES ACNC PT UR ORD SCREEN Amphets Ur Ql Scn 9426-8 BARBITURATES MCNC PT UR QN Barbiturates Ur-mCnc 16429-3 BARBITURATES ACNC PT UR ORD CONFIRM Barbiturates Ur Ql Cfm 19270-8 BARBITURATES ACNC PT UR ORD SCREEN Barbiturates Ur Ql Scn 3390-2 BENZODIAZEPINES ACNC PT UR ORD Benzodiaz Ur Ql 9428-4 BENZODIAZEPINES MCNC PT UR QN Benzodiaz Ur-mCnc 16195-0 BENZODIAZEPINES ACNC PT UR ORD CONFIRM Benzodiaz Ur Ql Cfm 14316-4 BENZODIAZEPINES ACNC PT UR ORD SCREEN Benzodiaz Ur Ql Scn 14315-6 BENZOYLECGONINE ACNC PT UR ORD CONFIRM BZE Ur Ql Cfm 14314-9 BENZOYLECGONINE ACNC PT UR ORD SCREEN BZE Ur Ql Scn 19289-8 CANNABINOIDS ACNC PT UR ORD CONFIRM Cannabinoids Ur Ql Cfm 18282-4 CANNABINOIDS ACNC PT UR ORD SCREEN Cannabinoids Ur Ql Scn 14639-9 CARBAMAZEPINE SCNC PT SER/PLAS QN CBZ SerPl-sCnc 3432-2 CARBAMAZEPINE MCNC PT SER/PLAS QN CBZ SerPl-mCnc 3398-5 COCAINE MCNC PT UR QN Cocaine Ur-mCnc 10365-5 COTININE MCNC PT SER/PLAS QN Cotinine SerPl-mCnc 12293-7 COTININE ACNC PT UR ORD Cotinine Ur Ql 9372-4 COTININE MCNC PT XXX QN Cotinine XXX-mCnc 3530-3 DELTA-9-TETRAHYDROCANNABINOL MCNC PT UR QN D9THC Ur-mCnc 19419-1 DEXTROAMPHETAMINE ACNC PT UR ORD SCREEN D-amphet Ur Ql Scn 10535-3 DIGOXIN MCNC PT SER/PLAS QN Digoxin SerPl-mCnc 14698-5 DIGOXIN SCNC PT SER/PLAS QN Digoxin SerPl-sCnc 14719-9 ETHANOL SCNC PT SER/PLAS QN Ethanol SerPl-sCnc 5639-0 ETHANOL ACNC PT BLD ORD Ethanol Bld Ql 5640-8 ETHANOL MCNC PT BLD QN Ethanol Bld-mCnc 5643-2 ETHANOL MCNC PT SER/PLAS QN Ethanol SerPl-mCnc 5644-0 ETHANOL ACNC PT UR ORD Ethanol Ur Ql 14334-7 LITHIUM SCNC PT SER/PLAS QN Lithium SerPl-sCnc 3719-2 LITHIUM MCNC PT SER/PLAS QN Lithium SerPl-mCnc 19528-9 LYSERGATE DIETHYLAMIDE ACNC PT UR ORD SCREEN LSD Ur Ql Scn 3774-7 METHADONE MCNC PT UR QN Methadone Ur-mCnc 16199-2 METHADONE ACNC PT UR ORD CONFIRM Methadone Ur Ql Cfm 19550-3 METHADONE ACNC PT UR ORD SCREEN Methadone Ur Ql Scn 19554-5 METHAMPHETAMINE ACNC PT UR ORD SCREEN Methamphet Ur Ql Scn 3787-9 METHAQUALONE MCNC PT UR QN Methaqualone Ur-mCnc 18389-7 METHAQUALONE ACNC PT UR ORD CONFIRM Methaqualone Ur Ql Cfm
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 9 of 16 LOINC® Fully Specified LOINC® Name LOINC® short name code Component Property Time System Scale Method Aspect 19558-6 METHAQUALONE ACNC PT UR ORD SCREEN Methaqualone Ur Ql Scn 8220-6 OPIATES MCNC PT UR QN Opiates Ur-mCnc 18390-5 OPIATES ACNC PT UR ORD CONFIRM Opiates Ur Ql Cfm 19295-5 OPIATES ACNC PT UR ORD SCREEN Opiates Ur Ql Scn 3937-0 PHENCYCLIDINE MCNC PT UR QN PCP Ur-mCnc 18392-1 PHENCYCLIDINE ACNC PT UR ORD CONFIRM PCP Ur Ql Cfm 19659-2 PHENCYCLIDINE ACNC PT UR ORD SCREEN PCP Ur Ql Scn 3954-5 PHENOTHIAZINE MCNC PT UR QN Phenothiaz Ur-mCnc 16221-4 PHENOTHIAZINE ACNC PT UR ORD CONFIRM Phenothiaz Ur Ql Cfm 19670-9 PHENOTHIAZINE ACNC PT UR ORD SCREEN Phenothiaz Ur Ql Scn 3545-1 PROPOXYPHENE MCNC PT UR QN Propoxyph Ur-mCnc 16200-8 PROPOXYPHENE ACNC PT UR ORD CONFIRM Propoxyph Ur Ql Cfm 19429-0 PROPOXYPHENE ACNC PT UR ORD SCREEN Propoxyph Ur Ql Scn 14915-3 THEOPHYLLINE SCNC PT SER/PLAS QN Theophylline SerPl-sCnc 4049-3 THEOPHYLLINE MCNC PT SER/PLAS QN Theophylline SerPl-mCnc 22752-0 TOBRAMYCIN^TROUGH SCNC PT SER/PLAS QN Tobramycin Tr SerPl-sCnc 4059-2 TOBRAMYCIN^TROUGH MCNC PT SER/PLAS QN Tobramycin Tr SerPl-mCnc 16181-0 TRICYCLIC ANTIDEPRESSANTS MCNC PT UR QN CONFIRM Tricyclics Ur Cfm-mCnc 19315-1 TRICYCLIC ANTIDEPRESSANTS ACNC PT UR ORD CONFIRM Tricyclics Ur Ql Cfm 19312-8 TRICYCLIC ANTIDEPRESSANTS ACNC PT UR ORD SCREEN Tricyclics Ur Ql Scn
HISTORY 8665-2 DATE LAST MENSTRUAL PERIOD TMSTP PT ^PATIENT QN REPORTED
HEMATOLOGY & CELL COUNTS 7789-1 ACANTHOCYTES ACNC PT BLD ORD MICROSCOPY.LIGHT Acanthocytes Bld Ql Smear 702-1 ANISOCYTOSIS ACNC PT BLD ORD MICROSCOPY.LIGHT Anisocytosis Bld Ql Smear 11281-3 AUER RODS ACNC PT BLD ORD MICROSCOPY.LIGHT Auer Bodies Bld Ql Smear 703-9 BASOPHILIC STIPPLING ACNC PT BLD ORD MICROSCOPY.LIGHT Baso Stipl Bld Ql Smear 26444-0 BASOPHILS NCNC PT BLD QN Basophils # Bld 30180-4 BASOPHILS/100 LEUKOCYTES NFR PT BLD QN Basophils % Bld 10371-3 BITE CELLS ACNC PT BLD ORD MICROSCOPY.LIGHT Bite Cells Bld Ql Smear 30376-8 BLASTS NCNC PT BLD QN Blasts # Bld 26446-5 BLASTS/100 LEUKOCYTES NFR PT BLD QN Blasts % Bld 7790-9 BURR CELLS ACNC PT BLD ORD MICROSCOPY.LIGHT Burr Cells Bld Ql Smear 11280-5 CABOT RINGS ACNC PT BLD ORD MICROSCOPY.LIGHT Cabot Rings Bld Ql Smear 4485-9 COMPLEMENT C3 MCNC PT SER/PLAS QN C3 SerPl-mCnc 4498-2 COMPLEMENT C4 MCNC PT SER/PLAS QN C4 SerPl-mCnc 4532-8 COMPLEMENT TOTAL HEMOLYTIC CH50 ACNC PT SER/PLAS QN CH50 SerPl-aCnc 7791-7 DACRYOCYTES ACNC PT BLD ORD MICROSCOPY.LIGHT Dacryocytes Bld Ql Smear 7792-5 DOHLE BODY ACNC PT BLD ORD MICROSCOPY.LIGHT Dohle Bod Bld Ql Smear 11274-8 ELLIPTOCYTES ACNC PT BLD ORD MICROSCOPY.LIGHT Elliptocytes Bld Ql Smear 26449-9 EOSINOPHILS NCNC PT BLD QN Eosinophil # Bld 26450-7 EOSINOPHILS/100 LEUKOCYTES NFR PT BLD QN Eosinophil % Bld 30385-9 ERYTHROCYTE DISTRIBUTION WIDTH RATIO PT RBC QN RDW RBC-Rto 28539-5 ERYTHROCYTE MEAN CORPUSCULAR HEMOGLOBIN ENTMASS PT RBC QN MCH RBC Qn 28540-3 ERYTHROCYTE MEAN CORPUSCULAR HEMOGLOBIN MCNC PT RBC QN MCHC RBC-mCnc CONCENTRATION 4537-7 ERYTHROCYTE SEDIMENTATION RATE VEL PT BLD QN WESTERGREN ESR Bld Qn Westrgrn 26453-1 ERYTHROCYTES NCNC PT BLD QN RBC # Bld 26461-4 ERYTHROCYTES.NUCLEATED/100 ERYTHROCYTES NFR PT BLD QN nRBC/RBC % Bld 19048-8 ERYTHROCYTES.NUCLEATED/100 LEUKOCYTES RATIO PT BLD QN nRBC/WBC Bld-Rto
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 10 of 16 LOINC® Fully Specified LOINC® Name LOINC® short name code Component Property Time System Scale Method Aspect 30397-4 HAIRY CELLS NCNC PT BLD QN Hairy Cells # Bld 24106-7 HAIRY CELLS/100 LEUKOCYTES NFR PT BLD QN MANUAL COUNT Hairy Cells % Bld Manual 4542-7 HAPTOGLOBIN MCNC PT SER QN Haptoglob Ser-mCnc 716-1 HEINZ BODIES ACNC PT BLD ORD MICROSCOPY.LIGHT Heinz Bod Bld Ql Smear 10374-7 HELMET CELLS ACNC PT BLD ORD MICROSCOPY.LIGHT Helmet Cells Bld Ql Smear 20570-8 HEMATOCRIT VFR PT BLD QN Hct % Bld 4545-0 HEMATOCRIT VFR PT BLD QN SPUN Hct % Bld Spun 14134-1 HEMOGLOBIN MCNC PT SER QN Hgb Ser-mCnc 25433-4 HEMOGLOBIN SCNC PT PLAS QN Hgb Plas-sCnc 718-7 HEMOGLOBIN MCNC PT BLD QN Hgb Bld-mCnc 20572-4 HEMOGLOBIN A/HEMOGLOBIN.TOTAL MFR PT BLD QN ELECTROPHORESIS Hgb A % Bld Elph 4547-6 HEMOGLOBIN A1/HEMOGLOBIN.TOTAL MFR PT BLD QN Hgb A1 % Bld 4548-4 HEMOGLOBIN A1C/HEMOGLOBIN.TOTAL MFR PT BLD QN Hgb A1c % Bld 17856-6 HEMOGLOBIN A1C/HEMOGLOBIN.TOTAL MFR PT BLD QN HPLC Hgb A1c % Bld HPLC 4562-5 HEMOGLOBIN C/HEMOGLOBIN.TOTAL MFR PT BLD QN ELECTROPHORESIS PH Hgb C % Bld Elph Alk 8.9 4625-0 HEMOGLOBIN S/HEMOGLOBIN.TOTAL MFR PT BLD QN Hgb S % Bld 7793-3 HOWELL-JOLLY BODIES ACNC PT BLD ORD MICROSCOPY.LIGHT Howell-Jolly Bod Bld Ql Smear 30400-6 HYPOCHROMIA ACNC PT BLD ORD Hypochromia Bld Ql 27415-9 INTERFERON.GAMMA MCNC PT SER/PLAS QN G-IFN SerPl-mCnc 13629-1 INTERLEUKIN 1 BETA MCNC PT SER/PLAS QN Il1Beta SerPl-mCnc 26848-2 INTERLEUKIN 10 MCNC PT SER/PLAS QN Il10 SerPl-mCnc 27161-9 INTERLEUKIN 4 MCNC PT SER/PLAS QN Il4 SerPl-mCnc 26881-3 INTERLEUKIN 6 MCNC PT SER/PLAS QN Il6 SerPl-mCnc 26462-2 LARGE UNSTAINED CELLS NCNC PT BLD QN LUC # Bld 26463-0 LARGE UNSTAINED CELLS/100 LEUKOCYTES NFR PT BLD QN LUC % Bld 26464-8 LEUKOCYTES NCNC PT BLD QN WBC # Bld 30405-5 LEUKOCYTES NCNC PT UR QN WBC # Ur 26474-7 LYMPHOCYTES NCNC PT BLD QN Lymphocytes # Bld 26477-0 LYMPHOCYTES.ATYPICAL NCNC PT BLD QN Atypical Lymphs # Bld 13046-8 LYMPHOCYTES.ATYPICAL/100 LEUKOCYTES NFR PT BLD QN Atypical Lymphs % Bld 26478-8 LYMPHOCYTES/100 LEUKOCYTES NFR PT BLD QN Lymphocytes % Bld 30422-0 LYMPHOMA CELLS NCNC PT BLD QN Lymphoma Cells # Bld 30423-8 LYMPHOMA CELLS/100 LEUKOCYTES NFR PT BLD QN Lymphoma Cells % Bld 30424-6 MACROCYTES ACNC PT BLD ORD Macrocytes Bld Ql 30428-7 MEAN CORPUSCULAR VOLUME ENTVOL PT RBC QN MCV RBC Qn 30433-7 METAMYELOCYTES NCNC PT BLD QN Metamyelocytes # Bld 28541-1 METAMYELOCYTES/100 LEUKOCYTES NFR PT BLD QN Metamyelocytes % Bld 30434-5 MICROCYTES ACNC PT BLD ORD Microcytes Bld Ql 26484-6 MONOCYTES NCNC PT BLD QN Monocytes # Bld 26485-3 MONOCYTES/100 LEUKOCYTES NFR PT BLD QN Monocytes % Bld 18314-5 MORPHOLOGY IMP PT BLD NAR Morphology Bld-Imp 30444-4 MYELOBLASTS NCNC PT BLD QN Myeloblasts # Bld 30445-1 MYELOBLASTS/100 LEUKOCYTES NFR PT BLD QN Myeloblasts % Bld 30446-9 MYELOCYTES NCNC PT BLD QN Myelocytes # Bld 26498-6 MYELOCYTES/100 LEUKOCYTES NFR PT BLD QN Myelocytes % Bld 26499-4 NEUTROPHILS NCNC PT BLD QN Neutrophils # Bld 26507-4 NEUTROPHILS.BAND FORM NCNC PT BLD QN Neuts Band # Bld 26508-2 NEUTROPHILS.BAND FORM/100 LEUKOCYTES NFR PT BLD QN Neuts Band % Bld 765-8 NEUTROPHILS.HYPERSEGMENTED ACNC PT BLD ORD MICROSCOPY.LIGHT Neuts Hyperseg Bld Ql Smear 30451-9 NEUTROPHILS.SEGMENTED NCNC PT BLD QN Neuts Seg # Bld
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 11 of 16 LOINC® Fully Specified LOINC® Name LOINC® short name code Component Property Time System Scale Method Aspect 26505-8 NEUTROPHILS.SEGMENTED/100 LEUKOCYTES NFR PT BLD QN Neuts Seg % Bld 18319-4 NEUTROPHILS.VACUOLATED ACNC PT BLD ORD MICROSCOPY.LIGHT Neuts Vac Bld Ql Smear 26511-6 NEUTROPHILS/100 LEUKOCYTES NFR PT BLD QN Neutrophils % Bld 774-0 OVALOCYTES ACNC PT BLD ORD MICROSCOPY.LIGHT Ovalocytes Bld Ql Smear 7795-8 PAPPENHEIMER BODIES ACNC PT BLD ORD MICROSCOPY.LIGHT Pappenheimer Bod Bld Ql Smear 18311-1 PELGER HUET CELLS ACNC PT BLD ORD MICROSCOPY.LIGHT Pelger Huet Cells Bld Ql Smear 30458-4 PLASMA CELLS NCNC PT BLD QN Plasma Cells # Bld 11117-9 PLASMA CELLS.IMMATURE/100 CELLS NFR PT MAR QN MICROSCOPY Imm Plasma Cells % Mar Micro 13047-6 PLASMA CELLS/100 LEUKOCYTES NFR PT BLD QN Plasma Cells % Bld 28542-9 PLATELET MEAN VOLUME ENTVOL PT BLD QN PMV Bld Qn 18312-9 PLATELET SATELLITISM ACNC PT BLD ORD MICROSCOPY.LIGHT Platelet Satel Bld Ql Smear 26515-7 PLATELETS NCNC PT BLD QN Platelet # Bld 5908-9 PLATELETS.GIANT ACNC PT BLD ORD MICROSCOPY.LIGHT Giant Platelets Bld Ql Smear 32146-3 PLATELETS.LARGE ACNC PT BLD ORD MICROSCOPY.LIGHT Large Platelets Bld Ql Smear 779-9 POIKILOCYTOSIS ACNC PT BLD ORD MICROSCOPY.LIGHT Poikilocytosis Bld Ql Smear 10378-8 POLYCHROMASIA ACNC PT BLD ORD MICROSCOPY.LIGHT Polychromasia Bld Ql Smear 30464-2 PROLYMPHOCYTES NCNC PT BLD QN Prolymphocytes # Bld 30465-9 PROLYMPHOCYTES/100 LEUKOCYTES NFR PT BLD QN Prolymphocytes % Bld 30466-7 PROMONOCYTES/100 LEUKOCYTES NFR PT BLD QN Promonycytes % Bld 26523-1 PROMYELOCYTES NCNC PT BLD QN Promyelocytes # Bld 26524-9 PROMYELOCYTES/100 LEUKOCYTES NFR PT BLD QN Promyelocytes % Bld 14196-0 RETICULOCYTES NCNC PT RBC QN Retics # RBC 17849-1 RETICULOCYTES/100 ERYTHROCYTES NFR PT RBC QN AUTOMATED COUNT Retics/100 RBC % Auto 7797-4 ROULEAUX ACNC PT BLD ORD MICROSCOPY.LIGHT Rouleaux Bld Ql Smear 800-3 SCHISTOCYTES ACNC PT BLD ORD MICROSCOPY.LIGHT Schistocytes Bld Ql Smear 801-1 SICKLE CELLS ACNC PT BLD ORD MICROSCOPY.LIGHT Sickle Cells Bld Ql Smear 7798-2 SMUDGE CELLS ACNC PT BLD ORD MICROSCOPY.LIGHT Smudge Cells Bld Ql Smear 802-9 SPHEROCYTES ACNC PT BLD ORD MICROSCOPY.LIGHT Spherocytes Bld Ql Smear 10380-4 STOMATOCYTES ACNC PT BLD ORD MICROSCOPY.LIGHT Stomatocytes Bld Ql Smear 10381-2 TARGET CELLS ACNC PT BLD ORD MICROSCOPY.LIGHT Targets Bld Ql Smear 803-7 TOXIC GRANULES ACNC PT BLD ORD MICROSCOPY.LIGHT Toxic Granules Bld Ql Smear
MICROBIOLOGY 22203-4 CLOSTRIDIUM TETANI AB.IGG ACNC PT SER QN C tetani IgG Ser-aCnc 13227-4 CORYNEBACTERIUM DIPHTHERIAE AB.IGG ACNC PT SER QN C diphtheriae IgG Ser-aCnc 13949-3 CYTOMEGALOVIRUS AB.IGG ACNC PT SER ORD EIA CMV IgG Ser Ql EIA 20575-7 HEPATITIS A VIRUS AB ACNC PT SER ORD Hep A Ab Ser Ql 22314-9 HEPATITIS A VIRUS AB.IGM ACNC PT SER ORD Hep A IgM Ser Ql 16933-4 HEPATITIS B VIRUS CORE AB ACNC PT SER ORD Hep Bc Ab Ser Ql 22319-8 HEPATITIS B VIRUS CORE AB.IGM ACNC PT SER QN Hep Bc IgM Ser-aCnc 29900-8 HEPATITIS B VIRUS DNA MCNC PT SER QN PROBE.AMP.SIG Hep B DNA Ser bDNA-mCnc 29615-2 HEPATITIS B VIRUS DNA NCNC PT SER/PLAS QN PROBE.AMP.TAR Hep B Viral Load SerPl PCR 22320-6 HEPATITIS B VIRUS LITTLE E AB ACNC PT SER ORD Hep Be Ab Ser Ql 13954-3 HEPATITIS B VIRUS LITTLE E AG ACNC PT SER ORD EIA Hep Be Ag Ser Ql EIA 22322-2 HEPATITIS B VIRUS SURFACE AB ACNC PT SER ORD Hep Bs Ab Ser Ql 5195-3 HEPATITIS B VIRUS SURFACE AG ACNC PT SER ORD Hep Bs Ag Ser Ql 5196-1 HEPATITIS B VIRUS SURFACE AG ACNC PT SER ORD EIA Hep Bs Ag Ser Ql EIA 7905-3 HEPATITIS B VIRUS SURFACE AG ACNC PT SER ORD NEUT Hep Bs Ag Ser Ql Nt 16128-1 HEPATITIS C VIRUS AB ACNC PT SER ORD Hep C Ab Ser Ql
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 12 of 16 LOINC® Fully Specified LOINC® Name LOINC® short name code Component Property Time System Scale Method Aspect 11259-9 HEPATITIS C VIRUS RNA ACNC PT SER/PLAS ORD PROBE.AMP.TAR Hep C RNA SerPl Ql PCR 20416-4 HEPATITIS C VIRUS RNA NCNC PT SER/PLAS QN PROBE.AMP.TAR Hep C Viral Load SerPl PCR 13248-0 HEPATITIS D VIRUS AB ACNC PT SER ORD Hep D Ab Ser Ql 7909-5 HERPES SIMPLEX VIRUS 1 AB.IGG ACNC PT SER QN HSV1 IgG Ser-aCnc 7912-9 HERPES SIMPLEX VIRUS 2 AB.IGG ACNC PT SER QN HSV2 IgG Ser-aCnc 19106-4 HERPES SIMPLEX VIRUS AB.IGG ACNC PT SER ORD HSV IgG Ser Ql 7917-8 HIV 1 AB ACNC PT SER ORD HIV1 Ab Ser Ql 20447-9 HIV 1 RNA NCNC PT SER/PLAS QN PROBE.AMP.TAR HIV1 Viral Load SerPl PCR 7918-6 HIV 1+2 AB ACNC PT SER ORD HIV1+2 Ab Ser Ql 7919-4 HIV 2 AB ACNC PT SER ORD HIV2 Ab Ser Ql 6447-7 LEGIONELLA PNEUMOPHILA AG ACNC PT UR ORD EIA L pneumo Ag Ur Ql EIA 21406-4 MYCOPLASMA PNEUMONIAE AB.IGM ACNC PT SER ORD M pneumo IgM Ser Ql 29675-6 PARVOVIRUS B19 AB.IGG ACNC PT SER ORD B19V IgG Ser Ql 10710-2 PLASMODIUM SP IDENTIFIED PRID PT BLD NOM THIN FILM Plasmodium Bld Thin Film 20507-0 REAGIN AB ACNC PT SER ORD RAPID TEST RPR Ser Ql 5370-2 STREPTOLYSIN O AB ACNC PT SER QN ASO Ab Ser-aCnc 6561-5 TREPONEMA PALLIDUM AB.IGG ACNC PT SER ORD T pallidum IgG Ser Ql 6562-3 TREPONEMA PALLIDUM AB.IGM ACNC PT SER ORD T pallidum IgM Ser Ql
SEROLOGY 11013-0 DNA DOUBLE STRAND AB TITR PT SER QN dsDNA Ab Titr Ser 5130-0 DNA DOUBLE STRAND AB ACNC PT SER QN dsDNA Ab Ser-aCnc 12277-0 DNA DOUBLE STRAND AB ACNC PT SER ORD FARR dsDNA Ab Ser Ql Farr 5131-8 DNA DOUBLE STRAND AB ACNC PT SER ORD IF dsDNA Ab Ser Ql IF 8061-4 NUCLEAR AB ACNC PT SER ORD ANA Ser Ql 14611-8 NUCLEAR AB PATTERN IMP PT SER NOM ANA Pat Ser-Imp 11572-5 RHEUMATOID FACTOR ACNC PT SER QN RF Ser-aCnc
SPECIMEN 5778-6 COLOR TYPE PT UR NOM Color Ur 4691-2 VISCOSITY VISC PT PLAS QN Viscosity Plas Qn
URINALYSIS 5766-1 AMMONIUM URATE CRYSTALS ACNC PT URNS ORD MICROSCOPY.LIGHT Amm Urate Cry UrnS Ql 25145-4 BACTERIA ACNC PT URNS ORD MICROSCOPY.LIGHT Bacteria UrnS Ql 5771-1 BILIRUBIN CRYSTALS ACNC PT URNS ORD MICROSCOPY.LIGHT Bilirub Cry UrnS Ql 5773-7 CALCIUM CARBONATE CRYSTALS ACNC PT URNS ORD MICROSCOPY.LIGHT Ca Carbonate Cry UrnS Ql 5774-5 CALCIUM OXALATE CRYSTALS ACNC PT URNS ORD MICROSCOPY.LIGHT CaOx Cry UrnS Ql 5775-2 CALCIUM PHOSPHATE CRYSTALS ACNC PT URNS ORD MICROSCOPY.LIGHT Ca Phos Cry UrnS Ql 24124-0 CASTS ACNC PT URNS ORD MICROSCOPY.LIGHT Casts UrnS Ql 9842-6 CASTS NARIC PT URNS QN MICROSCOPY.LIGHT.LPF Casts UrnS Qn LPF 5777-8 CHOLESTEROL CRYSTALS ACNC PT URNS ORD MICROSCOPY.LIGHT Cholest Cry UrnS Ql 32167-9 CLARITY TYPE PT UR NOM Clarity Ur 5782-8 CRYSTALS PRID PT URNS NOM MICROSCOPY.LIGHT Crystals UrnS 5784-4 CYSTINE CRYSTALS ACNC PT URNS ORD MICROSCOPY.LIGHT Cystine Cry UrnS Ql 25157-9 EPITHELIAL CASTS ACNC PT URNS ORD MICROSCOPY.LIGHT Epith Casts UrnS Ql 5786-9 EPITHELIAL CASTS NARIC PT URNS QN MICROSCOPY.LIGHT.LPF Epith Casts UrnS Qn LPF 20453-7 EPITHELIAL CELLS ACNC PT URNS ORD MICROSCOPY.LIGHT Epi Cells UrnS Ql 5787-7 EPITHELIAL CELLS NARIC PT URNS QN MICROSCOPY.LIGHT.HPF Epi Cells UrnS Qn HPF 12248-1 EPITHELIAL CELLS.RENAL ACNC PT URNS ORD MICROSCOPY.LIGHT Renal Epi Cells UrnS Ql 5807-3 ERYTHROCYTE CASTS NARIC PT URNS QN MICROSCOPY.LIGHT.LPF RBC Casts UrnS Qn LPF 14290-1 ERYTHROCYTES ACNC PT URNS QN MANUAL COUNT RBC UrnS Manual-aCnc
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 13 of 16 LOINC® Fully Specified LOINC® Name LOINC® short name code Component Property Time System Scale Method Aspect 13945-1 ERYTHROCYTES NARIC PT URNS QN MICROSCOPY.LIGHT.HPF RBC UrnS Qn HPF 5808-1 ERYTHROCYTES NCNC PT URNS QN MICROSCOPY.LIGHT.HPF RBC # UrnS HPF 20409-9 ERYTHROCYTES NCNC PT UR QN TEST STRIP RBC # Ur Strip 5788-5 FAT BODIES.OVAL NARIC PT URNS QN MICROSCOPY.LIGHT.HPF Oval Fat Bod UrnS Qn HPF 25159-5 FATTY CASTS ACNC PT URNS ORD MICROSCOPY.LIGHT Fatty Casts UrnS Ql 5789-3 FATTY CASTS NARIC PT URNS QN MICROSCOPY.LIGHT.LPF Fatty Casts UrnS Qn LPF 25160-3 GRANULAR CASTS ACNC PT URNS ORD MICROSCOPY.LIGHT Gran Casts UrnS Ql 5793-5 GRANULAR CASTS NARIC PT URNS QN MICROSCOPY.LIGHT.LPF Gran Casts UrnS Qn LPF 5794-3 HEMOGLOBIN ACNC PT UR ORD TEST STRIP Hgb Ur Ql Strip 5795-0 HIPPURATE CRYSTALS ACNC PT URNS ORD MICROSCOPY.LIGHT Hippurate Cry UrnS Ql 25162-9 HYALINE CASTS ACNC PT URNS ORD MICROSCOPY.LIGHT Hyaline Casts UrnS Ql 5796-8 HYALINE CASTS NARIC PT URNS QN MICROSCOPY.LIGHT.LPF Hyaline Casts UrnS Qn LPF 5798-4 LEUCINE CRYSTALS ACNC PT URNS ORD MICROSCOPY.LIGHT Leucine Cry UrnS Ql 5799-2 LEUKOCYTE ESTERASE ACNC PT UR ORD TEST STRIP WBC Est Ur Ql Strip 5821-4 LEUKOCYTES NARIC PT URNS QN MICROSCOPY.LIGHT.HPF WBC UrnS Qn HPF 12235-8 MICROSCOPIC OBSERVATION PRID PT URNS NOM MICROSCOPY.LIGHT Micro UrnS 8247-9 MUCUS ACNC PT URNS ORD MICROSCOPY.LIGHT Mucous Threads UrnS Ql 5802-4 NITRITE ACNC PT UR ORD TEST STRIP Nitrite Ur Ql Strip 12453-7 PHOSPHATE CRYSTALS.AMORPHOUS ACNC PT URNS ORD MICROSCOPY.LIGHT Amorph Phos Cry UrnS Ql 2887-8 PROTEIN ACNC PT UR ORD Prot Ur Ql 2888-6 PROTEIN MCNC PT UR QN Prot Ur-mCnc 5812-3 SULFONAMIDE CRYSTALS ACNC PT URNS ORD MICROSCOPY.LIGHT Sulfonamide cry UrnS Ql 8249-5 TRANSITIONAL CELLS ACNC PT URNS ORD MICROSCOPY.LIGHT Trans Cells UrnS Ql 5813-1 TRICHOMONAS VAGINALIS ACNC PT URNS ORD MICROSCOPY.LIGHT T vaginalis UrnS Ql 5814-9 TRIPLE PHOSPHATE CRYSTALS ACNC PT URNS ORD MICROSCOPY.LIGHT Tri-Phos Cry UrnS Ql 5815-6 TYROSINE CRYSTALS ACNC PT URNS ORD MICROSCOPY.LIGHT Tyrosine Cry UrnS Ql 5817-2 URATE CRYSTALS ACNC PT URNS ORD MICROSCOPY.LIGHT Urate Cry UrnS Ql 12454-5 URATE CRYSTALS.AMORPHOUS ACNC PT URNS ORD MICROSCOPY.LIGHT Amorph Urate Cry UrnS Ql 5819-8 WAXY CASTS NARIC PT URNS QN MICROSCOPY.LIGHT.LPF Waxy Casts UrnS Qn LPF 21033-6 YEAST.BUDDING ACNC PT URNS ORD Yeast Budding UrnS Ql
LOINC® Copyright Notice and License
The LOINC®(R) codes, LOINC®(R) Users' Guide, and LOINC®(R) database are copyright (c) 1995-2005, Regenstrief Institute, Inc. and the Logical Observation Identifiers Names and Codes (LOINC®) Committee. All rights reserved.
LOINC®(R) is a registered trademark of Regenstrief Institute, Inc.
Permission is hereby granted in perpetuity, without payment of license fees or royalties, to use, copy, or distribute the LOINC®(R) codes, LOINC®(R) Users' Guide, and the LOINC®(R) database for any commercial or non-commercial purpose (RELMA(R) computer program, RELMA(R) database, and RELMA(R) Users’ Guide, which are covered by a different license, are not included in this license), subject to the following terms and conditions:
1) To prevent the dilution of the purpose of the LOINC® codes and LOINC® database of providing a definitive standard for identifying clinical information in electronic reports, users shall not use the LOINC® Users' Guide, the LOINC® codes, and/or the LOINC® database for the purpose of developing or promulgating a different standard for identifying laboratory test results, diagnostic study reports, or clinical measurements and observations or orders for such entities in electronic reports and messages.
2) Users shall not change the meaning of any of the LOINC® codes. Users shall not change the name of, or any contents of, any fields in the LOINC® database. Users may add new fields to the LOINC® database to attach additional information to existing LOINC® records.
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 14 of 16
3) A user may delete records from the LOINC® database to deal with the user's local requirements. A user also may add new records to the LOINC® database to deal with the users' local requirements, provided that if new records are added, any new entry in the LOINC®_NUM field (field #1) of such new records must contain a leading alphabetic "X" so that the new codes and records cannot be confused with existing LOINC® codes or new LOINC® codes as they are defined in later releases of the LOINC® database. Records deleted or added by users to deal with local requirements are not reflected in the official LOINC® database maintained by the Regenstrief Institute and the LOINC® Committee. Users will also make reasonable efforts to submit requests to LOINC® for new records to cover observations that are not found in the LOINC® data base in order to minimize the need for X-codes.
4) LOINC® codes and other information from the LOINC® database may be used in electronic messages for laboratory test results and clinical observations such as HL7 ORU messages, without the need to include this LOINC® Copyright Notice and License or a reference thereto in the message (and without the need to include all fields required by Section 6 hereof). When the LOINC® code (from the LOINC®_NUM field) is included in the message, users are encouraged, but not required, to include the corresponding LOINC® short name (from the SHORTNAME field) in the message if the message provides a place for a text name representation of the code.
5) Users may make and distribute an unlimited number of copies of the unmodified LOINC® database. Each copy of the unmodified LOINC® database must include this LOINC® Copyright Notice and License, and must include the version number of the database. This LOINC® Copyright Notice and License must appear on every printed copy of the LOINC® database. Where the LOINC® database is distributed on a fixed storage medium (such as diskette or CD-ROM), a printed copy of this LOINC® Copyright Notice and License must be included on or with the storage medium, and a text file containing this information also must be stored on the storage medium in a file called "LOINC ®_license.txt". Where the LOINC® database is distributed via the Internet, this LOINC® Copyright Notice and License must be accessible on the same Internet page from which the LOINC® database is available for download.
6) Subject to Section 1 and the other restrictions hereof, users may incorporate portions of the LOINC® database into another laboratory test definition database or software program for distribution outside of the user's corporation or organization, provided that any such laboratory test definition database or software program includes the following fields reproduced in their entirety from the LOINC® database: LOINC®_NUM (field #1), COMPONENT (field #2), PROPERTY (field #3), TIME_ASPCT (field #4), SYSTEM (field #5), SCALE_TYP (field #6), METHOD_TYP (field #7), ANSWERLIST (field #18), STATUS (field #19), and SHORTNAME (field #59).Users are encouraged, but not required, to also include the RelatedNames2 (field #58) in any such database. Further description of these fields is provided in Appendix A of the LOINC® Users' Guide. Every copy of the LOINC® database incorporated into or distributed in conjunction with another database or software program must include the following notice:
This product includes all or a portion of the LOINC®(R) database, or is derived from the LOINC®(R) database, subject to a license from Regenstrief Institute, Inc. Your use of the LOINC® database and LOINC® codes also is subject to this license, a copy of which is available at http://www.LOINC®.org/license. The current complete LOINC® database and Users' Guide are available for download at http://www.regenstrief.org/LOINC®. The LOINC® database and LOINC® codes are copyright (c) 1995-2005, Regenstrief Institute, Inc. and the Logical Observation Identifiers Names and Codes (LOINC®) Committee. All rights reserved. THE LOINC® DATABASE IS PROVIDED AS IS." ANY EXPRESS OR IMPLIED WARRANTIES ARE DISCLAIMED, INCLUDING, BUT NOT LIMITED TO, THE IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE. LOINC®(R) is a registered trademark of Regenstrief Institute, Inc."
If the laboratory test definition database or software program containing the LOINC® database is distributed with a printed license, this statement must appear in the printed license. Where the laboratory test definition database or software program containing the LOINC® database is distributed on a fixed storage medium, a text file containing this information also must be stored on the storage medium in a file called "LOINC®_short_license.txt". Where the laboratory test definition database or software program containing the LOINC® database is distributed via the Internet, this information must be accessible on the same Internet page from which the product is available for download.
7) Use and distribution of the LOINC® codes and LOINC® database in ways that are not specifically discussed herein shall always be accompanied by the notice provided in Section 6 hereof. The guidelines for providing the notice that are contained in the last paragraph of Section 6 also shall apply. If a user has a question about whether a particular use of the LOINC ® codes and/or the LOINC® database is permissible, the user is invited to contact the Regenstrief Institute by e-mail at LOINC®@regenstrief.org.
8) An unlimited number of copies of the LOINC® Users' Guide may be made and distributed. This LOINC® Copyright Notice and License must appear verbatim on every electronic or printed copy of the LOINC® Users' Guide. The LOINC® Users' Guide may not be modified, nor may derivative works of the LOINC® Users' Guide be created, without the prior written
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 15 of 16 permission of the Regenstrief Institute, Inc. To request written permission, please contact LOINC®@regenstrief.org. The Regenstrief Institute retains the right to approve any modification to, or derivative work of, the LOINC® Users' Guide.
9) The names "Regenstrief," "Regenstrief Foundation," "Regenstrief Institute," and "LOINC® Committee" may not be used in a way which could be interpreted as an endorsement or a promotion of any product or service without prior written permission of the Regenstrief Institute, Inc. To request written permission, please contact LOINC ®@regenstrief.org.
10) DISCLAIMER: REGENSTRIEF INSTITUTE, INC. AND THE LOINC® COMMITTEE DO NOT ACCEPT LIABILITY FOR ANY OMISSIONS OR ERRORS IN THE LOINC® CODES, LOINC® USERS'GUIDE OR THE LOINC® DATABASE. THE LOINC® CODES, LOINC® USERS' GUIDE AND LOINC® DATABASE ARE PROVIDED "AS IS," WITHOUT WARRANTY OF ANY KIND. ANY EXPRESS OR IMPLIED WARRANTIES ARE DISCLAIMED, INCLUDING, BUT NOT LIMITED TO, THE IMPLIED WARRANTIES OF TITLE, NON-INFRINGEMENT, MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE OR WARRANTIES ARISING FROM A COURSE OF DEALING, TRADE USAGE,OR TRADE PRACTICE. FURTHER, NO WARRANTY OR REPRESENTATION IS MADE CONCERNING THE ACCURACY, COMPLETENESS, SEQUENCE, TIMELINESS OR AVAILABILITY OF THE LOINC® CODES, LOINC® USERS' GUIDE OR THE LOINC® DATABASE. IN NO EVENT SHALL REGENSTRIEF INSTITUTE, INC. OR THE LOINC® COMMITTEE OR ITS CONTRIBUTORS BE LIABLE FOR ANY DIRECT, INDIRECT, INCIDENTAL, SPECIAL, EXEMPLARY, RELIANCE, OR CONSEQUENTIAL DAMAGES OR ATTORNEYS’ FEES (INCLUDING, BUT NOT LIMITED TO, PROCUREMENT OF SUBSTITUTE GOODS OR SERVICES; OPPORTUNITY COSTS; LOSS OF USE, DATA, SAVINGS OR PROFITS; OR BUSINESS INTERRUPTION) HOWEVER CAUSED AND ON ANY THEORY OF LIABILITY WHETHER IN CONTRACT, STRICT LIABILITY, OR TORT (INCLUDING NEGLIGENCE OR OTHERWISE) ARISING IN ANY WAY OUT OF THE USE OF LOINC® CODES, LOINC® USERS'GUIDE OR THE LOINC® DATABASE, EVEN IF ADVISED OF THE POSSIBILITY OF SUCH DAMAGE. SOME JURISDICTIONS DO NOT ALLOW THE LIMITATION OR EXCLUSION OF CERTAIN WARRANTIES OR CONDITIONS, SO SOME OF THE FOREGOING MAY NOT APPLY TO YOU.
11) This license shall be construed and interpreted in accordance with the laws of the State of Indiana, United States of America, excluding its conflicts of law rules.
Copyright 1995-2005, Regenstrief Institute, Inc. and LOINC® Committee, All rights reserved. Page 16 of 16