Mr. Applebaum’s Second Marking Period Chemistry Project “ADOPT AN ELEMENT”
Requirements: 1 - Bibliography / Reference Page (30 pts) Due Date: ______2 - Element Fact Sheet (30 pts) Due Date: ______3 - Advertising Poster (40 pts) Due Date: ______My element: ______10% lost for each day late
1 - Bibliography / Reference Page: Your project begins with research. You must have at least three references. Your textbook is good place to start. You can also use magazines, encyclopedias (hard copy or on-line), books on CD and/or internet sites*. After you have found all the required information, you are to cite your specific references in proper form (Author, Title, Resource Location, Date). Check you student handbook (page R-7) to see how books, web pages, etc. are to be cited. I should be able to find the exact information you found by checking your references.
2 – Each section of the fact sheet on the backside of this page) must be as complete and neat as possible. For example, if your element has no discoverer, in that it has been known in antiquity, you are to write something like “known since ancient times – discoverer unknown”. Costs must be converted to one of the three options listed and common isotopes are hereby defined as those that are stable along with those with a half life no less than a year.
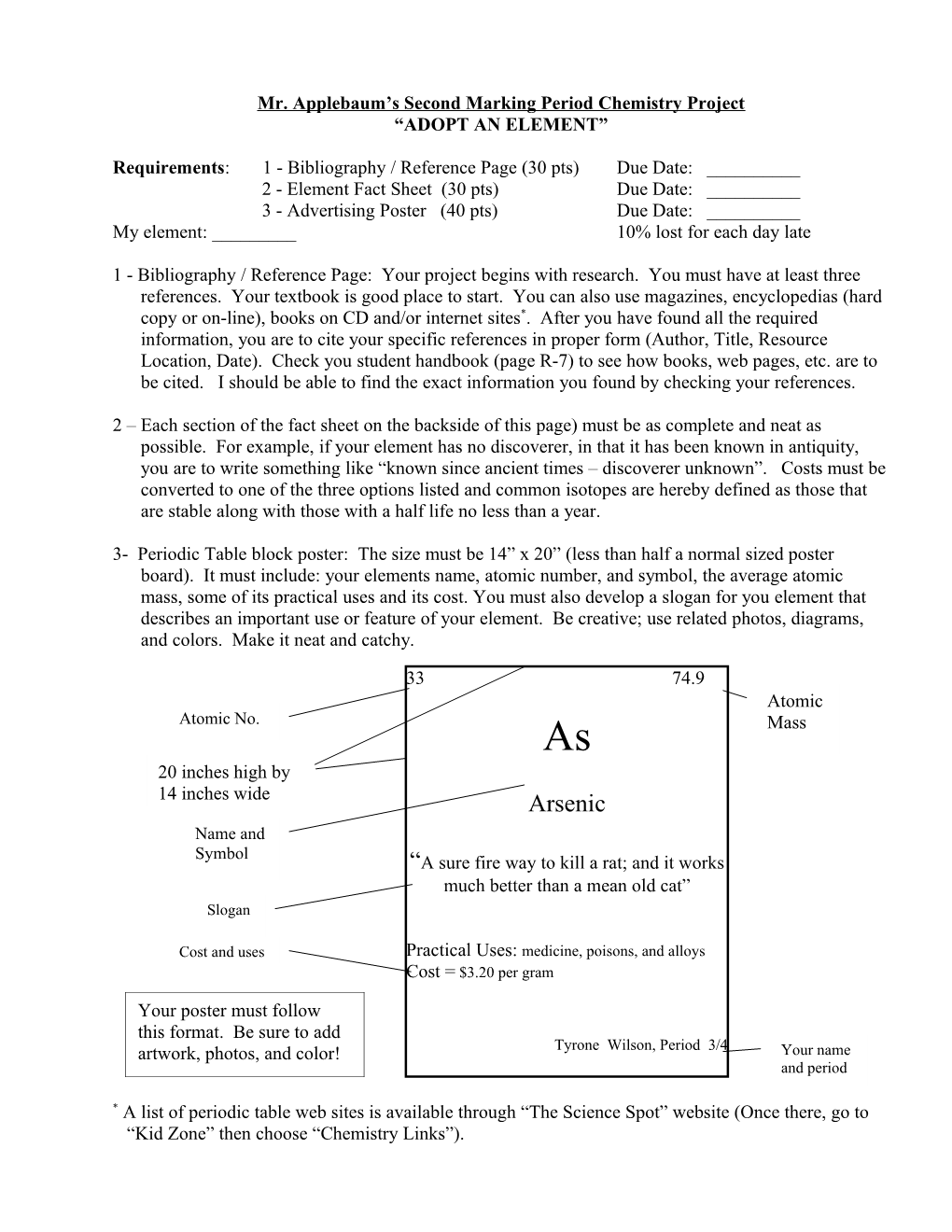
3- Periodic Table block poster: The size must be 14” x 20” (less than half a normal sized poster board). It must include: your elements name, atomic number, and symbol, the average atomic mass, some of its practical uses and its cost. You must also develop a slogan for you element that describes an important use or feature of your element. Be creative; use related photos, diagrams, and colors. Make it neat and catchy.
33 74.9 Atomic Atomic No. As Mass 20 inches high by 14 inches wide Arsenic Name and Symbol “A sure fire way to kill a rat; and it works much better than a mean old cat” Slogan
Cost and uses Practical Uses: medicine, poisons, and alloys Cost = $3.20 per gram
Your poster must follow this format. Be sure to add artwork, photos, and color! Tyrone Wilson, Period 3/4 Your name and period
* A list of periodic table web sites is available through “The Science Spot” website (Once there, go to “Kid Zone” then choose “Chemistry Links”). ”Adopt an Element” Student name: ______Per. ______Fact Sheet Element name: ______
Symbol: ______Atomic Number: ______Average Atomic Mass: ______
Symbols of the common isotopes: ______
Electron Configuration: [ Closed Shell ] ______Valence Electrons: ______
Period number: ______Group number: ______Group Name: ______
Melting point: ______C Boiling point: ______C Normal state (or phase) ______
Cost data (1 required): $ ______per gram, $ ______per pound, $ ______per ton
Discovered by ______in ______[year]
Origin of the modern name: ______
______
Other names used in history: ______
Interesting info.: [may include important uses, manufacturing facts, common compounds, etc…]
1. ______
______
2. ______
______
3. ______
______
4. ______
______
5. ______
______
6. ______
______”Adopt an Element” Student name: ______Per. ______Bibliography / Element name: ______Reference Page (staple this page to your fact sheet)
1. ______
______
2. ______
______
3. ______
______
______
______
”Adopt an Element” Student name: ______Per. ______Bibliography / Element name: ______Reference Page (staple this page to your fact sheet)
1. ______
______
2. ______
______
3. ______
______
______
______“My Element” Sign Up Sheet Period: ______
No. Element Student Name 1 Aluminum, Al 2 Americium, Am 3 Argon, Ar 4 Barium, Ba 5 Beryllium, Be 6 Bismuth, Bi 7 Boron, B 8 Bromine, Br 9 Cadmium, Cd 10 Calcium, Ca 11 Carbon, C 12 Cesium, Cs 13 Chlorine, Cl 14 Chromium, Cr 15 Cobalt, Co 16 Copper, Cu 17 Fluorine, F 18 Gold, Au 19 Helium, He 20 Hydrogen, H 21 Iodine, I 22 Iron, Fe 23 Krypton, Kr 24 Lead, Pb 25 Lithium, Li 26 Magnesium, Mg 27 Manganese, Mn 28 Mercury, Hg 29 Neon, Ne 30 Nickel, Ni 31 Nitrogen, N 32 Oxygen, O 33 Phosphorus, P 34 Platinum, Pt 35 Plutonium, Pu 36 Potassium, K 37 Radium, Ra 38 Radon, Rn 39 Silicon, Si 40 Silver, Ag 41 Sodium, Na 42 Strontium, Sr 43 Sulfur, S 44 Tin, Sn 45 Titanium, Ti 46 Tungsten, W 47 Uranium, U 48 Xenon, Xe 49 Zinc, Zn 50 Zirconium, Zr