Gateway 125,126,130 Fall 2006 Final Exam p1
Gateway General Chemistry 125/126/130 Final Exam December 19, 2006 (8:00-10:00am)
Name______KEY______
Section (circle one): 601 (Colin) 602 (Brannon) 603 (Mali) 604 (Xiaomu)
The exam has at total of 10 pages including the cover, a periodic table, and a table of standard reduction potentials. You may remove the last two pages and do not need to turn them in with your exam. Please neatly show all of your work and apply significant figure rules. The cover contains any formulas or conversions you may need.
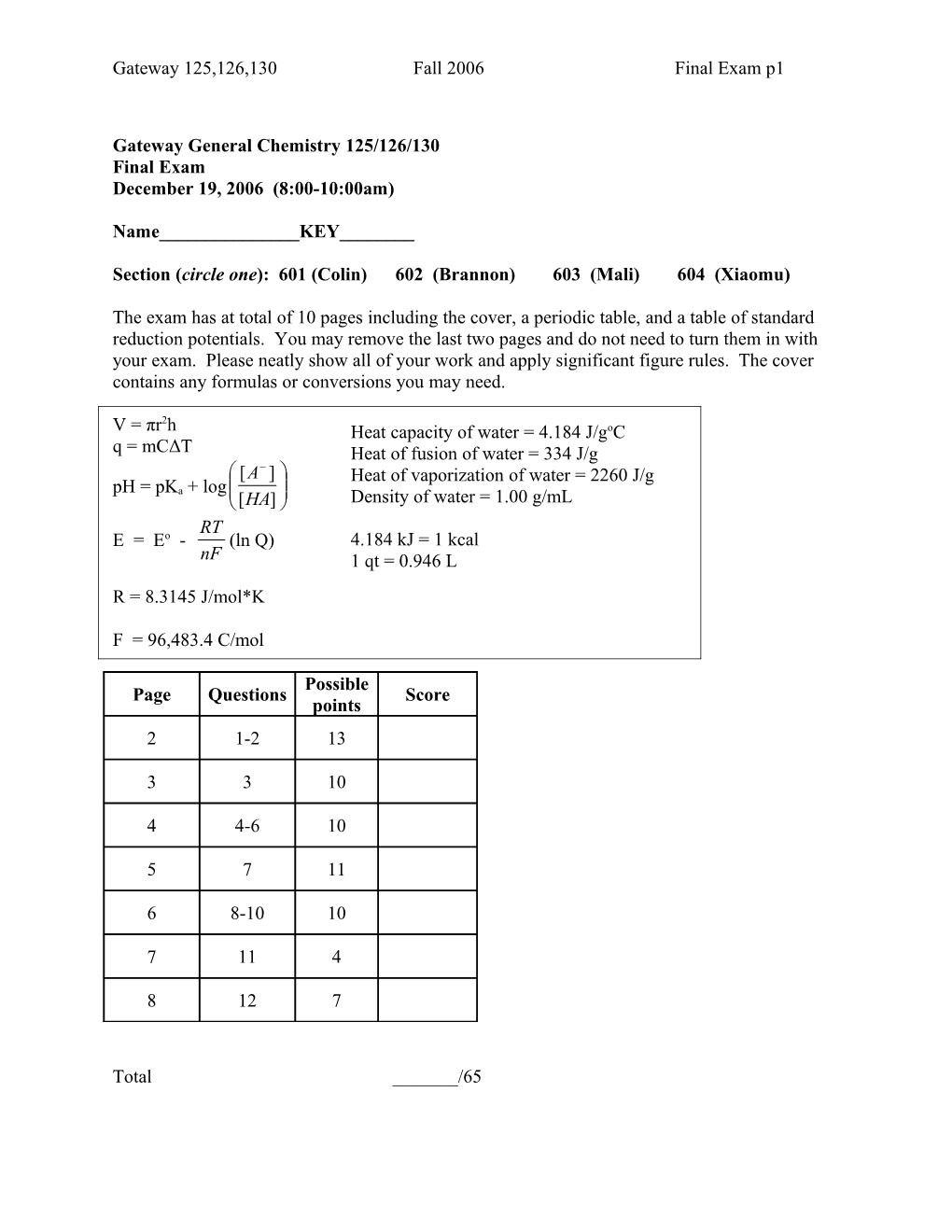
2 V = πr h Heat capacity of water = 4.184 J/goC q = mCΔT Heat of fusion of water = 334 J/g [A ] Heat of vaporization of water = 2260 J/g pH = pKa + log [HA] Density of water = 1.00 g/mL RT E = Eo - (ln Q) 4.184 kJ = 1 kcal nF 1 qt = 0.946 L R = 8.3145 J/mol*K
F = 96,483.4 C/mol
Possible Page Questions Score points
2 1-2 13
3 3 10
4 4-6 10
5 7 11
6 8-10 10
7 11 4
8 12 7
Total ______/65 Gateway 125,126,130 Fall 2006 Final Exam p2
1a) (1 point) Write out the electron b) (1 point) If bismuth forms an anion, it configuration for bismuth (Bi). You may use would most likely to gain how many the abbreviated noble gas configuration. electrons? 3
[Xe]6s24f145d106p3 c) (3 points) 209Bi has how many protons? neutrons? electrons? 83 126 83 d) (1 point) Bismuth would have e) (1 point) e) Bismuth would have an an ionization energy that is (circle one) electronegativity that is (circle one)
greater than, less than, or equal to greater than, less than, or equal to
the ionization energy of nitrogen. the electronegativity of astatine (At). f) (2 points) On the diagram g) (2 points) For each of the electrons that you included in below, draw in the 6p orbitals of part f, write in a possible quantum NUMBER for each Bi. Place the correct number of column in the table. The table may or may not be filled. electrons for bismuth into each orbital. n l ml ms y 6 1 0 +1/2 6 1 -1 +1/2 6 1 1 +1/2 x
z
2) (2 points) In the middle column, write one of the symbols: <, > or =. (In order to receive credit for your answer, all estimation and work must be shown.) The number of carbon atoms in 100 mg 8x1032 carbon atoms. of fructose C6H12O6 <
100 mg 1g 1 mol 6x1023 molecules 1000mg 80 g 1 mol
= 3.34x1020 * 6 C in 1 molecule = 2 x1021
The abundance of 35Cl in a natural sample The abundance of 37Cl in a natural sample of chlorine. > of chlorine.
75% of 35.5 g/mol Gateway 125,126,130 Fall 2006 Final Exam p3
3a) (2.5 points) For the molecule BF3: i) count the number of valence electrons: 24
ii) Draw the Lewis structure; iii) Draw the 3-D VSEPR shape iv) Name the electron pair include any formal charges: along with an arrow indicating geometry: the net polarity of the molecule trigonal planar F if any: F B F v) Name the molecular B F geometry: trigonal planar F F
3b) (2.5 points) For the molecule CF4: i) count the number of valence electrons: 32
ii) Draw the Lewis structure; iii) Draw the 3-D VSEPR shape iv) Name the electron pair include any formal charges: along with an arrow indicating geometry: the net polarity of the molecule tetrahedral F if any: F F C F F v) Name the molecular C F geometry: tetrahedral F F
3c) (2.5 points) For the molecule NF3: i) count the number of valence electrons: 26
ii) Draw the Lewis structure; iii) Draw the 3-D VSEPR shape iv) Name the electron pair include any formal charges: along with an arrow indicating geometry: the net polarity of the molecule tetrahedral F if any:
N F v) Name the molecular N geometry: F F trigonal pyramidal F F
3d) (2.5 points) For the molecule OF2: i) count the number of valence electrons: 20
ii) Draw the Lewis structure; iii) Draw the 3-D VSEPR shape iv) Name the electron pair include any formal charges: along with an arrow indicating geometry: the net polarity of the molecule tetrahedral O if any: O F F v) Name the molecular F F geometry: bent Gateway 125,126,130 Fall 2006 Final Exam p4
4) (2 points) Given the reaction: SrO(s) + CO2(g) SrCO3(s) H = -234 kJ
a) This reaction is (circle one) endothermic exothermic
b) What would be the ΔH of the reaction if 0.5 g of SrO was consumed? 0.5 g SrO 1 mol SrO -234 kJ 103.62 g SrO 1 mol SrO = -1.04 kJ = -1 kJ
5) (4 points) A website makes the following claim: a 1 oz. (28.4 g) piece of sweet chocolate provides 144 kcal (144,000 calories). Accordingly, there is sufficient energy in 1 oz. of chocolate to boil 1.50 qt of "ice cold" water (0oC).
Verify the claim. q = mCΔT 1.5 qt 0.9461 L 1000 mL 1 g 4.184 J 100-0 oC 1 qt 1 L 1 mL goC
=593.7 kJ 144 kcal(* 4.184 kJ/kcal) = 602.5 kJ > 593.7 kJ
What mass of water could be boiled with the extra energy in the chocolate bar? 602.5 kJ- 593.7 kJ = 8.8 kJ (1000 J/kJ) = x g(2260 J/g) 3.89 g = x 3.89 mL = 4 mL
6a) (3 points) Find the heat of reaction for the chemical equation C(s) + 2H2(g) → CH4(g) Given the following reactions: 1) C(s) + O2(g) → CO2(g) ΔH= –394 kJ/mol 2) H2(g) + ½ O2(g) → H2O(l) ΔH= –286 kJ/mol 3) CH4(g) + 2O2(g) →CO2(g) +2 H2O(l) ΔH= –891 kJ/mol
C(s) + O2(g) → CO2(g) ΔH= –394 kJ/mol 2H2(g) + O2(g) → 2H2O(l) ΔH=2( –286 kJ/mol) CO2(g) +2 H2O(l) → CH4(g) + 2O2(g) Δ H= 891 kJ/mol C(s) + 2H2(g) → CH4(g) ΔH = -75 kJ/mol
6b) (1 point) Which of the numbered reactions in part 6a represent a heat of formation? (Circle the number/s if any) 1 2 3 Gateway 125,126,130 Fall 2006 Final Exam p5
- 7) The EPA has set a MCL of nitrite (NO2 ) at 3.3 ppm nitrite. a) (1 point) What is this concentration of nitrite in molarity? - - 3.3 mg NO2 1 g 1 mol NO2 - L 1000 mg 46 g NO2 -5 - = 7.2 x10 M NO2 b) (1 point) Nitrite is (circle one): a strong acid weak acid strong base weak base ?
c) (2 points) Based on your answer above, write the equilibrium of nitrite with water. Identify the acid, the base, the conjugate acid, and the conjugate base. - - NO2 (aq) + H2O(l) ↔ HNO2(aq) + OH (aq) Base acid conj. acid conj. base
-4 d) (3 points) What would be the pH of a 0.35 M solution of nitrite? Ka HNO2 = 4.47 x10 - - NO2 ↔ H NO2 OH I 0.35 0 0 C -x +x +x E 0.35-x x x -14 -4 -11 Kb = Kw/Ka = 1x10 /4.47x10 = 2.24 x10 - = [H NO2 ][OH ] - [NO2 ] = x2/(0.35) 2.8x10-6 = x = [OH-] pOH = -log[OH-] = -log[2.8x10-6] = 5.6 pH = 14-5.6 = 8.4 e) (4 points) You need to prepare 1.00 L of a buffer that contains 0.50 moles of nitrite. Which of the following could you add to 0.50 moles nitrite to prepare the buffer? If the choice would make a good buffer, fill in the pH of the buffer in the second column. Buffer with nitrite? If yes, then pH of resulting buffer. (circle one)
0.25 moles of NaOH Yes No
- NO2 = HNO2 0.25 moles of HCl Yes No pH = pKa = 3.3
0.60 moles of HCl Yes No
- pH = pKa + log[A ]/[HA] = 3.3 + log(0.5/0.4) = 3.4 0.40 moles HNO2 Yes No Gateway 125,126,130 Fall 2006 Final Exam p6
8) (4 points) If you look up how to remove soap scum (Ca(OH)2(s) and Mg(OH)2(s)) in Haley’s Hints, it suggests using vinegar [(4% acetic acid (CH3COOH)].
a) Write the equilibrium for the dissolution of Ca(OH)2 in water. +2 - Ca(OH)2(s) ↔ Ca (aq) + 2OH (aq)
b) Write the equilibrium expression (Keq):
+2 - 2 Keq =[Ca ][OH ]
c) Explain how vinegar helps to dissolve soap scum.
- - CH3COOH + OH → CH3COO + H2O Vinegar reacts with hydroxide (a strong base) to form acetate. As OH- is removed from the solubility expression, more Ca(OH)2 is dissolved
9) (2 points) Rank the following in order of increasing oxidation number of S in each species: -2 H2S, S8, SCl2, SO3 , K2SO4
-2 H2S < S8 < SCl2 < SO3 < K2SO4 lowest oxidation number highest oxidation number
10) (4 points) Balance the following redox reactions. Identify the oxidizing agent and the + - reducing agent. Use H3O or OH in your final reaction.
+2 +4 ¯ H2O2(aq) + Ti (aq) → H2O(l) + Ti (aq) in acid NO2(aq) + Al(s) → NH3(aq) + AlO2 (aq) in base
- + + - 2e + H2O2 + 2H → 2 H2O 7H + NO2 + 7 e → NH3 + 2H2O +2 +4 - + - Ti → Ti + 2 e 2H2O + Al → AlO2 + 4H + 4e
+2 + +4 H2O2 + Ti + 2 H3O → 4H2O + Ti 6 H2O + 4 NO2 + 7 Al → 4NH3 + 7 AlO2
- oxidizing agent: H2O2 oxidizing agent: NO2 reducing agent: Ti+2 reducing agent: Al Gateway 125,126,130 Fall 2006 Final Exam p7
11) (4 points) Balance each chemical reaction and calculate the Standard Cell Potential using the table of Standard Reduction Potentials. Will the forward chemical reaction spontaneously occur?
Will Forward Standard Cell Potential Reaction Occur? Circle one.
+ +2 Yes No 2.37 + 0 = 2.37 Mg(s) + 2 H3O (aq) → Mg (aq) + H2(g) + 2H2O(l)
+ - Yes No 2.714+ 1.08 = 3.79 2 Na(s) + Br2(l) → 2 Na (aq) + 2 Br (aq)
+2 +1 Yes No -0.80 + 0.337 = -0.46 2 Ag(s) + Cu (aq) → 2 Ag (aq) + Cu(s)
+ + Yes No 3.045-2.714 = 0.331 Li(s) + Na (aq) → Li (aq) + Na(s) Gateway 125,126,130 Fall 2006 Final Exam p8
12a) (5 points) Using the table of standard reduction potentials, make a battery with the highest standard potential using two metals and their 1.0M aqueous ion solutions. (Keep in mind that all metals from magnesium below react rapidly with water as shown in class and are not suitable choices.) Write in the correct metals for each the anode and the cathode, show the direction of electron flow, write in what enters each solution from the salt bridge, and calculate standard cell potential.
Direction of electron flow
anode metal cathode metal Al Au
KNO3
Species from Species from salt bridge salt bridge - + NO3 K
Standard potential 3.16V
12b) (2 points) What would the potential of your battery be if 0.5 M solutions were used at both the anode and cathode and the battery was operating at 60oC.
RT E = Eo - (ln Q) nF = 3.16 – RT (ln (0.5/0.5)) nF
= 3.16 – RT (0) nF
= 3.16