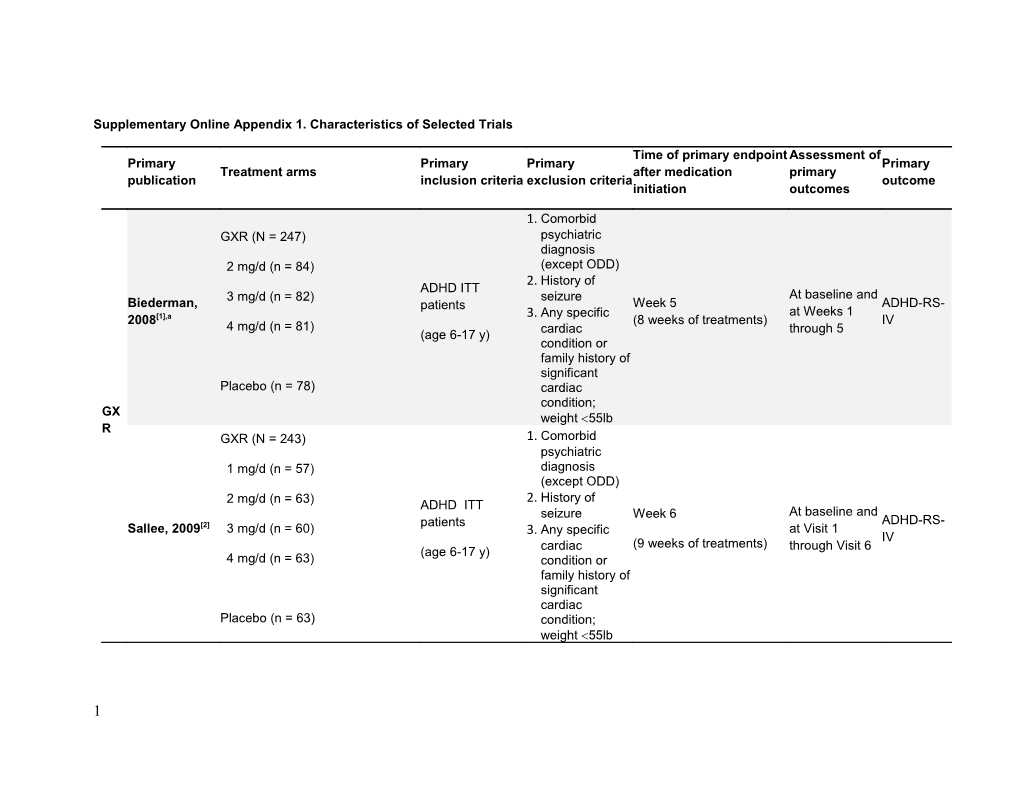
Supplementary Online Appendix 1. Characteristics of Selected Trials
Time of primary endpoint Assessment of Primary Primary Primary Primary Treatment arms after medication primary publication inclusion criteria exclusion criteria outcome initiation outcomes
1. Comorbid GXR (N = 247) psychiatric diagnosis 2 mg/d (n = 84) (except ODD) History of ADHD ITT 2. 3 mg/d (n = 82) seizure At baseline and Biederman, patients Week 5 ADHD-RS- [1],a 3. Any specific at Weeks 1 2008 4 mg/d (n = 81) (8 weeks of treatments) IV (age 6-17 y) cardiac through 5 condition or family history of significant Placebo (n = 78) cardiac condition; GX weight 55lb R GXR (N = 243) 1. Comorbid psychiatric 1 mg/d (n = 57) diagnosis (except ODD) History of 2 mg/d (n = 63) ADHD ITT 2. seizure Week 6 At baseline and [2] patients ADHD-RS- Sallee, 2009 3 mg/d (n = 60) 3. Any specific at Visit 1 (9 weeks of treatments) IV (age 6-17 y) cardiac through Visit 6 4 mg/d (n = 63) condition or family history of significant cardiac Placebo (n = 63) condition; weight 55lb
1 ATX 1. Serious medical illness, a history ADHD ITT of psychosis or ATX 1.8 mg/kg/d patients (age 6- bipolar disorder 12 y) with ADHD- 2. Alcohol or drug (n = 126) RS-IV symptom abuse within Weeks 1, 4, ADHD-RS- Kelsey, 2004[3] the past 3 Week 8 severity restriction IV months, and and 8 (1.5 SDs above ongoing use of Placebo (n = 60) age and gender psychoactive normative values) medications other than the study medication ADHD ITT 1. Poor ATX 2 mg/kg/d patients (age 7- metabolizers of 12 y) with ADHD- CYP2D6 Every week of Spencer, (n = 129) RS-IV symptom 2. Weight 55 lb the study from ADHD-RS- Week 9 2002[4] severity restriction 3. History of the baseline for IV (1.5 SDs above bipolar I or II both the studies Placebo (n = 124) age and gender disorder or any normative values) history of psychosis Michelson, ATX 1.5 mg/kg/d ADHD ITT 1. Serious medicalWeek 6 Weeks 1, 2, 4, ADHD-RS- 2002[5] patients (age 6-16 illness, history and 6 IV (n = 85) y) with ADHD-RS- of psychosis or IV symptom bipolar disorder severity restriction 2. Alcohol or drug abuse, or (1.5 SDs above Placebo (n = 85) ongoing use of age and gender psychoactive normative values) medications other than the study medication
2 1.IQ 80 ATX Serious medical ADHD ITT 2. illness, 0.5 mg/kg/d (n = 44) patients (age 8-18 comorbid y) with ADHD-RS- psychosis or 1.2 mg/kg/d (n = 84) Michelson, IV symptom history of a Weeks 1, 2, 3, ADHD-RS- Week 8 [6] severity restriction seizure 2001 1.8 mg/kg/d (n = 85) 4, 6, and 8 IV (1.5 SDs above 3.Ongoing use of age and gender psychoactive normative values) medications Placebo (n = 84) other than the study medication a Sample sizes were from unpublished clinical trial data.
GXR = guanfacine extended release; ADHD = attention-deficit/ hyperactivity disorder; ITT = intention-to-treat; ODD = oppositional defiant disorder; ADHD-RS-IV = ADHD Rating Scale IV; ATX = atomoxetine; SD = standard deviation; CYP2D6 = cytochrome P450 2D6.
Sikirica V, Findling RL, Signorovitch J, Erder MH, Dammerman R, Hodgkins P, Lu M, Xie J, Wu EQ. Comparative efficacy of guanfacine extended release versus atomoxetine for the treatment of attention-deficit/hyperactivity disorder in children and adolescents: applying matching-adjusted indirect comparison methodology.
3 Supplementary Online Appendix References
(1) Biederman J, Melmed RD, Patel A, et al. A randomized, double-blind, placebo-controlled study of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. Pediatrics 2008 Jan; 121 (1): e73-e84.
(2) Sallee FR, McGough J, Wigal T, Donahue J, Lyne A, Biederman J. Guanfacine extended release in children and adolescents with attention- deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry 2009 Feb; 48 (2): 155-65.
(3) Kelsey DK, Sumner CR, Casat CD, et al. Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics 2004 Jul; 114 (1): e1-e8.
(4) Spencer T, Heiligenstein JH, Biederman J, et al. Results from 2 proof-of-concept, placebo-controlled studies of atomoxetine in children with attention-deficit/hyperactivity disorder. J Clin Psychiatry 2002 Dec; 63 (12): 1140-7.
(5) Michelson D, Allen AJ, Busner J, et al. Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002 Nov; 159 (11): 1896-901.
(6) Michelson D, Faries D, Wernicke J, et al. Atomoxetine in the treatment of children and adolescents with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled, dose-response study. Pediatrics 2001 Nov; 108 (5): E83.
4