CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
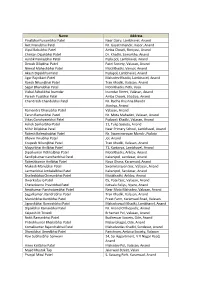
Members – List.Pdf
Name Address Pinalbhai Punambhai Patel Near Dairy, Lambhavel, Anand Axit Manubhai Patel Nr. Gayatrimandir, Kasor, Anand Vipul Babubhai Patel Amba Chowk, Boriyavi, Anand Chintan Dipakbhai Patel Dr. Khadki, Samarkha, Anand Hardik Pankajbhai Patel Pipla pol, Lambhavel, Anand Denish Dilipbhai Patel Patel Society, Valasan, Anand Nirmal Maheshbhai Patel Moti Khadki, Vansol, Anand Akash Dipakbhai Patel Piplapol, Lambhavel, Anand Jigar Rajnikant Patel Mahadev Khadki, Lambhavel, Anand Ronak Nikunjbhai Patel Tran Khadki, Valasan, Anand Sagar Bhanubhai Patel Moti Khadki, Petli, Vaso Vishal Ashokbhai Inamdar Inamdar Street, Valasan, Anand Paresh Pujabhai Patel Amba Chowk, Jitodiya, Anand Chandresh Chandubhai Patel Nr. Radha Krushna Mandir Jitodiya, Anand Ramendra Dhanjibhai Patel Valasan, Anand Tarun Ramanbhai Patel Nr. Mota Mahadev, Valasan, Anand Vikas Ganshyambhai Patel Piplavali Khadki, Valasan, Anand Ashok Sankarbhai Patel 11, Tulip Society, Anand Mihir Dilipbhai Patel Near Primary School, Lambhavel, Anand Rakesh Balendrabhai Patel Nr. Swaminarayan Mandir, Piplata Bhavin Vinubhai Patel Jol, Anand Krupesh Nikunjbhai Patel Tran Khadki, Valasan, Anand Mayurbhai Anilbhai Patel 71, Kartavya, Lambhavel, Anand Dipalkumar Vithhalbhai Patel Moti Khadki, Anklav, Anand. Sandipkumar Kanchanbhai Patel Kakanipol, sandesar, Anand Rakeshkumar Anilbhai Patel Nava Ghara, Karamsad, Anand Mukesh Manubhai Patel Swaminarayan Soc, Valasan, Anand Laxmanbhai Ambalalbhai Patel Kakanipol, Sandesar, Anand Shaileshbhai Chimanbhai Patel Motikhadki, Anklav, Anand Dwarkadas -

Madhya Gujarat Vij Company Ltd. Division Office, Petlad
MADHYA GUJARAT VIJ COMPANY LTD. DIVISION OFFICE, PETLAD NR-RAILWAY CROSSING, NADIAD-PETLAD RD Phone No. 02697-224936 /Fax No. 02697-223300 Web Site : www.mgvcl.com /www.gseb.com Tender Nivida : 01/15 Tender Notice No:EE (O&M)/Petlad/ –Khabmhat city SDn/ 2014/ FOR Laying of 11 KV U/G XLPE cable& O/H line work SE (O&M) Anand invites on line tender for “Laying of 11 KV U/G XLPE cable& O/H line work under Sagar khedu (CADP)Scheme.” from Registered Contractors in appropriate class with MGVCL/Central/State Government / Railway/Semi. Govt. and who has executed similar nature of work and magnitude successfully. Technical terms & conditions are available on web site URL www.gseb.com”Tenders-MGVCL-Corporate Office” and www.mgvcl.com and www.nprocure.com ‘Tender ID No _______” It is compulsory for all bidders to submit their tender/s documents by both forms i.e. online & physical within schedule time .If Tender is submitted in any one form i.e. either by on line OR physically, the bid shall not be considered. Note: Be in touch with above websites till opening of tender. Sr. Name of Estimated Time Tender E.M.D. No. Work Limit Cost Rs. Fee Rs. Rs. 01. Laying of U/G XLPE cable for 11 KV UG Rs. 2 1000/-(Non Rs. Cable 25,54,367/- Months refundable) 25,550/- work under RAPDRP Scheme. 1) Last date & time of physical receipt of tender:(Technical bids): 23.01.15 up to 16.00 hrs. 2) Last date of on-line Tender submission: 21.01.15 up to18.00 hrs. -

Smt. S. I. Patel Ipcowala College of Education, Petlad
Smt. S. I. Patel Ipcowala College of Education, Petlad. (Accredited by NAAC: “B” Grade with 2.58) (Managed by The Petlad Education Trust, Petlad.) College Campus, Dantali Road, PETLAD – 388 450 Dist. Anand, Gujarat, India Tel.: 02697 – 252228 www. bedcollegepetlad. org Email: [email protected] The Annual Quality Assurance Report AQAR of the IQAC 2008-2009 0 The Annual Quality Assurance Report (AQAR) of the IQAC Name of the Institution : Smt. S. I. Patel Ipcowala College of Education, Petlad. Office Address : College Campus, Dantali Road, Petlad – 388 450 Dist. Anand, (Guj.). Name of the Head of the : Dr. Anilkumar G. kachhia. Institution Phone No. : (Office) 02697 - 252228, 223228 (Resident) 02697 - 252428 (Mobile) 9998969728 College Website : www.bedcollegepetlad.org . Email : [email protected] Name of the IQAC coordinator : Dr. Natavarlal M. Solanki Phone No. : (Resident) 02697 - 235865 (Mobile) 9913247807 1 SMT. S. I. PATEL IPCOWALA COLLEGE OF EDUCATION, PETLAD. The establishment of Internal Quality Assurance Cell ( IQAC ) The Composition of the IQAC is as under: 1. Chairperson : Dr. A. G. kachhia ( Principal ) 2. Administrative Officers : Shri C. D. Shah 3. Teachers : Shri Y. R. Parmar Dr. J. V. Patel Smt. N. T. Shukla 4. Members from the management : Shri H. M. Shah Shri S. N. Kachhia 5. Local Society : Dr. Rajesh K. Trivedi Shri Jayesh K. Patel 6. Coordinator : Dr. N. M. Solanki 2 Part - A The plan of action chalked out by the IQAC in the beginning of the year towards quality enhancement and the outcome achieved by the end of the year. Brief outline of the Action Plan (2008-2009) The IQAC of the college prepared a complete plan at the beginning of the year to be followed all through out the academic year 2008-2009. -
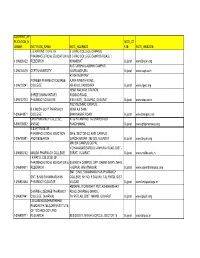
Current Ap Plication Umber N Institute Name Insti Address Insti St Ate Insti Website 1-396085422 L B Rao Institute of Pharmac
CURRENT_AP PLICATION_N INSTI_ST UMBER INSTITUTE_NAME INSTI_ADDRESS ATE INSTI_WEBSITE L B RAO INSTITUTE OF B D RAO COLLEGE CAMPUS, PHARMACEUTICAL EDUCATION & B D RAO COLLEGE CAMPUS ROAD, 1-396085422 RESEARCH KHAMBHAT Gujarat www.lbriper.org KASTURBHAI LALBHAI CAMPUS 1-396100415 CEPT UNIVERSITY NAVRANGPURA Gujarat www.cept.ac.in AT-SAYAJIPYRA PIONEER PHARMACY DEGREE AJWA-NIMETA ROAD 1-396103241 COLLEGE NR-N.H.8, VADODARA Gujarat www.ogect.org NEAR RAILWAY STATION, SHREE DHANVANTARY KUDSAD ROAD 1-396137213 PHARMACY COLLEGE KIM (EAST), TA-OLPAD, DI-SURAT Gujarat www.sdpc.co.in POLYTECHNIC CAMPUS, B.K.MODY GOVT.PHARMACY NEAR AJI DAM 1-396649871 COLLEGE BHAVNAGAR ROAD Gujarat www.bkmgpc.com GHB PHARMACY COLLEGE, AT & PO-ANIYAD,,, TA-SHAHERA,DI- 1-396708382 ANIYAD PANCHMAHAL Gujarat www.ghbpharmacy.org K.B.INTITUTE OF PHARMACEUTICAL EDUCTION GH/6, SECTOR-23, KADI CAMPUS, 1-396780981 AND RESEARCH GANDHINAGAR- 382 023, GUJARAT Gujarat www.kbiper.org MALIBA CAMPUS,GOPAL VIDYANAGAR,BARDOLI-MAHUVA ROAD, DIST - 1-396882142 MALIBA PHARMACY COLLEGE SURAT, GUJARAT Gujarat www.maliba.edu.in I.K.PATEL COLLEGE OF PHARMACEUTICAL EDUCATION & SAMARTH CAMPUS, OPP. SABAR DAIRY, NH-8, 1-396899501 RESEARCH HAJIPUR, HIMATNAGAR Gujarat www.samarthcampus.com SMT. B.N.B. SWAMINARAYAN PHARMACY SMT. B.N.B SWAMINARAYAN COLLEGE, NH NO. 8 SALVAV, TAL PARDI, DIST 1-396901664 PHARMACY COLLEGE VALSAD Gujarat www.bnbspcollege.in AMRAPALI TOWNSHIP, PETLAD-KHAMBHAT DHARMAJ DEGREE PHARMACY ROAD, DHARMAJ-388430 1-396907441 COLLEGE, DHARMAJ TA: PETLAD, DIST: ANAND, GUJARAT Gujarat www.ipcprc.org LEELABEN DASHRATHBHAI RAMDAS PATEL(LDRP)INSTITUTE OF TECHNOLOGY AND 1-396908711 RESEARCH BESIDES ITI, NR KH 5 CIRCLE, SECTOR 15 Gujarat www.ldrp.ac.in KRISHNA KAMPUS, BECHRAJI-SHANKHALPUR SHREE KRISHNA INSTITUTE OF ROAD, TALUKA-BECHRAJI, DIST-MEHSANA, 1-396910173 PHARMACY GUJARAT Gujarat www.skip.org.in MEHSANA - VISNAGAR HIGHWAY, AT & PO. -
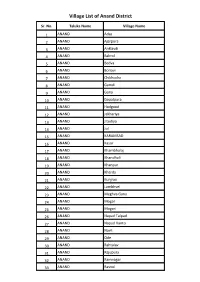
Village List of Anand District
Village List of Anand District Sr. No. Taluka Name Village Name 1 ANAND Adas 2 ANAND Ajarpura 3 ANAND Anklavdi 4 ANAND Bakrol 5 ANAND Bedva 6 ANAND Boriavi 7 ANAND Chikhodra 8 ANAND Gamdi 9 ANAND Gana 10 ANAND Gopalpura 11 ANAND Hadgood 12 ANAND Jakhariya 13 ANAND Jitodiya 14 ANAND Jol 15 ANAND KARAMSAD 16 ANAND Kasor 17 ANAND Khambholaj 18 ANAND Khandhali 19 ANAND Khanpur 20 ANAND Kherda 21 ANAND Kunjrao 22 ANAND Lambhvel 23 ANAND Meghva Gana 24 ANAND Mogar 25 ANAND Mogari 26 ANAND Napad Talpad 27 ANAND Napad Vanto 28 ANAND Navli 29 ANAND Ode 30 ANAND Rahtalav 31 ANAND Rajupura 32 ANAND Ramnagar 33 ANAND Rasnol 34 ANAND Samarkha 35 ANAND Sandesar 36 ANAND Sarsa 37 ANAND Sundan 38 ANAND Tarnol 39 ANAND Vadod 40 ANAND Vaghasi 41 ANAND Vaherakhadi 42 ANAND Valasan 43 ANAND Vans Khiliya 44 ANAND Vasad Village List of Petlad Taluka Sr. No. Taluka Name Village Name 1 PETLAD Agas 2 PETLAD Amod 3 PETLAD Ardi 4 PETLAD Ashi 5 PETLAD Bamroli 6 PETLAD Bandhani 7 PETLAD Bhalel 8 PETLAD Bhatiel 9 PETLAD Bhavanipura 10 PETLAD Bhurakui 11 PETLAD Boriya 12 PETLAD Changa 13 PETLAD Dantali 14 PETLAD Danteli 15 PETLAD Davalpura 16 PETLAD Demol 17 PETLAD Dhairyapura 18 PETLAD Dharmaj 19 PETLAD Fangani 20 PETLAD Ghunteli 21 PETLAD Isarama 22 PETLAD Jesarva 23 PETLAD Jogan 24 PETLAD Kaniya 25 PETLAD Khadana 26 PETLAD Lakkadpura 27 PETLAD Mahelav 28 PETLAD Manej 29 PETLAD Manpura 30 PETLAD Morad 31 PETLAD Nar 32 PETLAD Padgol 33 PETLAD Palaj 34 PETLAD Pandoli 35 PETLAD Petlad 36 PETLAD Porda 37 PETLAD Ramodadi 38 PETLAD Rangaipura 39 PETLAD Ravipura 40 PETLAD Ravli 41 PETLAD Rupiyapura 42 PETLAD Sanjaya 43 PETLAD Sansej 44 PETLAD Shahpur 45 PETLAD Shekhadi 46 PETLAD Sihol 47 PETLAD Silvai 48 PETLAD Simarada 49 PETLAD Sunav 50 PETLAD Sundara 51 PETLAD Sundarana 52 PETLAD Vadadala 53 PETLAD Vatav 54 PETLAD Virol(Simarada) 55 PETLAD Vishnoli 56 PETLAD Vishrampura Village List of Borsad Taluka Sr. -

Mandatory Disclosure 2020-21
CHAROTAR UNIVERSITY OF SCIENCE AND TECHNOLOGY (CHARUSAT) FACULTY OF MANAGEMENT STUDIES (FMS) INDUKAKA IPCOWALA INSTITUTE OF MANAGEMENT 2 (I IM) Mandatory Disclosure 2020-21 Mandatory Disclosure Updated on July 11, 2020. AICTE file No. Central/1-7011879849/2020/EOA Date & Time Period June 13, 2020. 1 Name of the Institution INDUKAKA IPCOWALA INSTITUTE OF MANAGEMENT Address of the Institution Education Campus AT PO: CHANGA TA: Petlad, DIST: Anand PIN:388421, CHANGA, ANAND, Gujarat, 388421, City & Pin Code Changa - 388421 State Gujarat Longitude & Latitude Longitude: 73. Latitude: 22 Phone No. 02697-247500 Fax No. 02697-247100 Office Hours at the Institution 9:00 am to 4:30 pm Academic hours at the 9:10 am to 4:20 pm Institution Email [email protected] Website www.charusat.ac.in Nearest Railway Station(dist. Nadiad Railway Station (11 Km) in km) Nearest Airport (dist. in km) Vadodara Airport (60 Km) Type of Institution Private- Self Financed Category (1) of the Institution Non Minority 2 Name and address of the SHREE CHAROTAR MOTI SATTAVIS Trust/ Society/ Company and PATIDAR KELAVANI MANDAL, ANAND the Trustees Type of organization Trust Address of the organization Vidhya Vihar Marg, B/H. Vaibhav Tower, Anand - Vidhyanagar Road, Anand Registered with Collector, Petlad Sub Registrar Registration Date 23/12/1998 3 Name of the Principal Prof. (Dr) Govind B Dave Exact Designation Principal Phone Number with STD code Mobile: +91-9099015370 Fax Number with STD code 02697-265007 Email [email protected] Highest Degree Ph. D. (Doctorate) Field of specialization Management 4 Name of the affiliating Charotar University of Science & University Technology CHARUSAT Campus-Changa Off. -

List of Colleges Under VV Nagar 1 Sankul
List Of Colleges Under VV Nagar 1 Sankul Sr. No College Code Institute Name Address Institute No. GTU. No Category GTU Email ID Inst Email ID A.D.PATEL INST.OF TECHNOLOGY,KARAMSAD P. O. Box No. 52, Vitthal Udyognagar, New 1 1 2692233680 9099063001 BE bec001owner@gtu. edu.in [email protected] (SFI) Vallabh Vidhyanagar, Dist. Anand - 388121. Sardar Vallabhbhai Patel Institute Of Technology 2 41 B/H S.T. Depot, VASAD - 388306 2692274766 9099063041 BE [email protected] [email protected] (SVIT), Vasad Institute Of Computer & Communication Beyond GIDC Phase-IV, New V.V. Nagar - 3 63 2692230824 9099063063 BE [email protected] [email protected] Technology For Women, New V.V.Nagar 388121. Revenue Block No.99, Vill : Mogar, NH# 8, 4 82 Dr. Jivraj Mehta Institute of Technology, Anand 2692280038 9099063481 BE [email protected] [email protected] Mogar, Anand 388340 5 205 Anand Pharmacy College, Anand (1st Shift) Nr. Town Hall, Anand 388001 2692250020 9099063105 BP [email protected] [email protected] 6 205 Anand Pharmacy College, Anand (2nd Shift) Nr. Town Hall, Anand 388001 2692250020 9099063105 BP [email protected] [email protected] Indukaka Ipcowala College Of Pharmacy, New B/h. GIDC,Phase-IV, New Vallabh 7 223 2765231800 9099063132 MP [email protected] [email protected] V.V.Nagar Vidyanagar - 388 121. Dharmaj Degree Pharmacy College, Dharmaj(1st Amrapali Township, Petlad-Khambat Road, 8 241 2697245808 919099063141 BP MP [email protected] [email protected] shift) Post : Dharmaj, Dist. Anand. Dharmaj Degree Pharmacy College, Dharmaj(2nd Amrapali Township, Petlad-Khambat Road, 9 241 2697245808 919099063141 BP MP [email protected] [email protected] shift) Post : Dharmaj, Dist. -

Survey of Angiospermic Weeds of Petlad Taluka, Anand District (Gujarat) India
The World Journal of Engineering & Applied Science ISSN 2349-4514 ICV Impact Factor 2.05 SURVEY OF ANGIOSPERMIC WEEDS OF PETLAD TALUKA, ANAND DISTRICT (GUJARAT) INDIA Article Received on Patel Kamlesh S. & Patel Kaushik C. P. G. Centre in Botany, Smt. S. M. Panchal Science 9 Mar 2017 College, Talod - 383215 Dist. Sabarkantha, North Gujarat, India Email: [email protected] Accepted on: 22 Apr 2017 ABSTRACT The present paper deals with some Angiospermic weeds of Petlad taluka. Weeds are known as unwanted plants. Usually weeds grow faster than native plants and successfully compete for the available nutrients, water, space and sunlight. It consists of total 22 families belonging 34 genera and 40 species. The dominant families were Euphorbiaceae with 5 species followed by Asteraceae with 4 species. A list of the Botanical names, Families, Common names, FLS and FRS were given in present paper. Key Words: Petlad taluka, Angiospermic weeds. INTRODUCTION Petlad Taluka is situated in Anand district nearby Borasd Taluka. Anand district is situated in the middle of Gujarat state, India. Anand district was established in the year 1997. The district of Anand comprises of 8 Talukas, i.e. Umareth, Petlad, Sojitra, Borsad, Anklav, Khambhat and Tarapur. Its population is about 2,092,745 people. It is located 21 km towards west from district Anand and 100 km far from state capital Gandhinagar towards North. Petlad Taluka is bounded by Borsad Taluka towards South, Sojitra Taluka towards North, Tarapur Taluka towards west, Khambhat Taluka towards south. Such Taluka has 57 villages. Petlad has been a major industrial contributor in the history of Gujarat. -

All College List GIA-SF
SARDAR PATEL UNIVERSITY AFFILIATED COLLEGES 1 Principal 2 Principal Anand Mercantile College of Science, AIMS College of Management & Technology Management & Computer Technology, AIMS Education Campus, Bhalej Road, Vadtal Road, Anand - 388 001. (SFI) Bakrol – 388 001. (SFI) 3 Principal 4 Principal Akhilesh Sureshbhai Patel Arts College, Anand Arts College, At. & Po. Boriavi – 387 310 Sh. Ramkrishna S eva Mandal , Ta. & Dist. Anand (SFI) Nr. Grid, Anand - 388 001 (GIA/SFI) 5 Principal 6 Principal Anand College of Education, Anand Commerce College, Sh. Ramkrishna Seva Mandal, Sh. Ramkrishna Seva M andal, Nr. Town Hall, Anand - 388 001. (SFI) Nr. Town Hall, Anand - 388 001. (GIA/SFI) 7 Principal 8 Principal Anand Education College, Anand Homoeopathic Medical College & Sh. Ramkrishna Seva Mandal, Research Institute, Sh. Ramkrishna Seva Nr. Town Hall, Mandal, Nr. Sardar Baug, Anand - 388 001. (GIA) Anand - 388 001 (GIA) 9 Principal 10 Principal Anand Institute of P.G. Studies In Arts, Anand Institute of Business Studies, Sh. Ramkrishna Seva Mandal, Sh. Ramkrishna Seva Mandal, Nr. Grid, Anand - 388 001 (SFI) Nr. Town Hall, Anand - 388 001. (SFI) 11 Principal 12 Principal Anand Institute of Social Work, Anand Law College, Sh. Ramkrishna Seva Mandal, Sh. Ramkrishna Seva Mandal, Nr. Town Hall, Nr. Town Hall , Anand - 388 001. (SFI) Anand - 388 001. (GIA/SFI) 13 Principal 14 Principal Ashok & Rita Patel Institute of Integrated Study Bhikhabhai Jivabhai Vanijya Mahavidyalaya &Research In Biotechnology And Allied Sciences (BJVM), (ARIBAS), Opp. Vitthal Udyognagar-GIDC, Vallabh Vidyanagar - 388 120. (GIA/SFI) Behind Phase-IV GIDC, New Vallabh Vidyanagar - 388 121. (SFI) 15 Principal 16 Principal B.N. -

The Role of Helping Hands in Industrial Development from Stakeholders’ Perception: a Survey Conducted at Vitthal Udyonagar in Anand District of Gujarat State, India
ISSN: 2319-8753 International Journal of Innovative Research in Science, Engineering and Technology (An ISO 3297: 2007 Certified Organization) Vol. 3, Issue 10, october 2014 The Role of Helping Hands in Industrial Development from Stakeholders’ Perception: A Survey Conducted At Vitthal Udyonagar in Anand District of Gujarat State, India T.B. Pankhania1, V.K. Modi2 Dr T.B.Pankhania Principal Sardar Patel college of Engineering, Bakrol Gujarat Technological University Gujarat India Lecturer, Department of Mechanical Engineering, B. &B. Institute of Technology, Vallabh Vidyanagar, Anand, Gujarat, India. ABSTRACT: In this paper the role of various government agencies is studied. The estate under consideration is situated in Anand district of Gujarat state, India. Government of Gujarat is helping in multidimensional way to the industrialists of the state as well. In this case, various aspects were studied to draw conclusions and have organization‟s effectiveness leading to highest industrial productivity and hence profitability. The main objective is to assess to what extent industrialist getting and / or availing the know-how including financing resources from all these agencies and to what extent they help moving growth faster than ever. In this way Industrial performance will improve and organization will generate more revenues and hence improve profitability. To test whether, there exists any correlation between government agencies and industrial growth of this area. Higher industrial growth with higher productivity, profitability, will improve living standard of the people. The industrialists will also become economically sound will get motivated for further business and expansion of the existing businesses due to healthy industrial organizations and conducive working environment of the surroundings. -

Paid Pending Applications As on Aug'19
PAID PENDING APPLICATIONS AS ON AUG'19 Circle: Anand Tentative Work Involved Sr Date Of Reg FQ Issue Date FQ Paid Date Month Of Scheme Sub Div Division Village Taluka District Name Load cat Remark No Work HT LT T/C Complition DD MM YYYY KM KM Cap DD MM YYYY DD MM YYYY V.V.NAGA 1 SPA Anand City Bakrol Anand Anand Malek Murtujahusen ayubmiya 10 8 10 2018 C 0.19 16 to 25 22 7 2019 23 7 2019 Sep-19 R V.V.NAGA 2 SPA Anand City Bakrol Anand Anand Patel Kanubhai Jashbhai 10 19 11 2018 B 0.025 0 22 7 2019 29 7 2019 Sep-19 R V.V.NAGA 3 SPA Anand City Bakrol Anand Anand Khureshi Asarafmiya Hasumiya 10 29 11 2018 C 0.17 10 to 16 22 7 2019 1 8 2019 Sep-19 R V.V.NAGA ANAND 4 SPA BAKROL Anand Anand PATEL JYOTIBEN NILESHBHAI 10 18 12 2018 D 0.08 10 KVA 22 7 2019 24 7 2019 Oct-19 R CITY 5 SPA Anand-S ANAND Khanpur Anand Anand Bhagvansinh Vakhatsinh Chakabhai Chauhan 10 18 5 2018 D 0.17 10 KVA 17 5 2019 11 6 2019 Sep-19 6 SPA Anand-S Anand Chikhodra Anand Anand Vasantbhai Ambalal Patel 15 2 7 2018 C 25 to 63 26 8 2019 27 8 2019 Sep-19 7 SPA Umreth-R Anand Chunel Mahudha Kheda Ravjibhai Bhikhabhai Prajapati 10 3 7 2018 D 0.63 10 KVA 25 6 2019 27 6 2019 Nov-19 Way problem 8 SPA Umreth-R Anand Parvata Umreth Anand Kesharbhai Chunabhai Rathod 7.5 9 7 2018 C 0.05 16 to 25 25 6 2019 11 7 2019 Sep-19 Standing paddy 9 SPA Umreth-R Anand Chunel Mahudha Kheda Pravinbhai Bhikhabhai Patel 7.5 19 7 2018 C 0.3 10 to 16 25 6 2019 2 7 2019 Nov-19 crop.photographs taken. -

List of Category Wise - District Wise Ulbs - Gujarat State Sr
List of Category wise - District wise ULBs - Gujarat State Sr. Name of Municipal Name of Municipalities No. District Corporation A B C D Total Ahmedabad Dholka Sanand Bareja 6 1 Ahmedabad Surat Viramgam Bavla Vadodara Dhandhuka Rajkot Amreli Rajula Lathi 9 Bhavnagar Savarkundla Bagasara Babara 2 Amreli Jamnagar Jafrabad Chalala Junagadh Damnagar Gandhinagar Anand Khambhat Umreth Anklav 11 Borsad Vallabh - Oad 3 Anand Vidhyanagar Petlad Karamsad Boriavi Sojitra 4 Arvalli Modasa Bayad 2 Palanpur Deesa Tharad 6 Dhanera 5 Banaskantha Bhabhar Thara 6 Bharuch Bharuch Ankleshwar Jambusar Amod 4 Mahuva Shihor Vallbhipur 6 7 Bhavnagar Palitana Gariyadhar Talaja 8 BOTAD Botad GADHADA BARVALA 3 CHHOTAUDEP 9 CHHOTAUDEPUR 1 UR Dahod Zalod Devgadh 10 Dahod 3 Bariya OKHA DWARKA BHANVAD 6 11 Devbhumi Dwarka JAMKHAMBHALIYA RAVAL(JAM) Salaya D:\Mahesh\ALL CEO\LIST OF CEO Distwise Classwise 1 Sr. Name of Municipal Name of Municipalities No. District Corporation A B C D Total Kalol Dehgam Pethapur 4 12 Gandhinagar Mansa Veraval-Patan Una Kodinar Talala 5 13 Geer Somnath SUTRAPADA Kalawad 4 Dhrol 14 Jamnagar Jamjodhpur Sikka Kesod Manavadar Chorvad 7 Mangrol Visavadar 15 Junagadh Vanthli Bantva Nadiad Kapadvanj Kheda 10 Chaklasi Dakor Mahemdavad Kathalal 16 Kheda Mahudha Kanjari Thasra Gandhidham Bhuj Mandvi Rapar 6 17 Kachchh Anjar Bhachau Mehsana Visnagar Vadnagar Vijapur 7 18 Mehsana Kadi Kheralu Unjha LUNAVADA SANTRAMPUR 3 19 Mahisagar BALASINOR MORBI WANKANER MALIYA-MIYANA 4 20 Morbi HALVAD 21 Narmada Rajpipla 1 D:\Mahesh\ALL CEO\LIST OF CEO Distwise