Iron Sequestration in Lake Sediments from Artificial Hypolimnetic Oxygenation: Richard B. Russell Reservoir Amanda Elrod Clemson University, [email protected]
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2009 Volume XXI, Number 1 Association, Inc
Lake Hartwell Winter, 2009 Volume XXI, Number 1 Association, Inc Letter from the President Inside this issue Submitted by Joe Brenner LHA’s Annual Fall Meeting 2 Lake Hartwell reached a record low level in October, and there’s no relief Drew Much Attention in sight. If the current drought continues, the entire conservation pool (625 LHA Represented at Historical 3 MSL) will be consumed by the end of 2009. The effects of climate change Water Conference are upon us. Though no one is sure how overall average rainfall will be affected in the southeastern U.S., all the climatologists I’ve heard from Hartwell Lake Level Projec- 4 tions (or, When will the lake have projected greater weather extremes, i.e. longer and more severe fill up again?) droughts. Ask the Corps 6 The existing Corps Drought Contingency Plan clearly cannot handle the 2008 Hartwell Lake Clean Up 7 weather patterns that we are experiencing. It is based on historical events Campaign a Success and decades old operational approaches. There must be a greater under- standing by all stakeholders within the Savannah River Basin that the reserves in the lakes must be Let’s Get Ready for Boating 8 Next Year maintained in order to protect the entire basin through severe drought situations. It must also be recognized that an appropriate drought plan will promote a “share the pain” approach throughout Proposed Nuclear Power 9 the basin. It is absurd to be holding boat races on the river in Augusta while our businesses suffer, Plant Expansion On The Savannah River our boat ramps are closed, the lake is not navigable and our docks sit on dirt. -

LHA News Fall 05
Lake Hartwell Fall, 2005 Volume XVII, Number 4 Association, Inc Letter from the President Inside this issue Submitted by Mike Massey 2005 Fall Informational 2 It has been a relatively beautiful summer on the lake. I hope you have all enjoyed it. Meeting The LHA Fall Meeting has been scheduled. Please take a minute to read about it and Anderson Co. Parks 3 when you have finished, mark your calendars to be sure you don’t miss out on this in- Benefit from formative annual event. Bioengineering LHA Annual Fall Meeting. Legislative Committee 4 The LHA Board of Directors is happy to announce that the Lake Hartwell Association Update annual meeting will be held on Thursday, November 10, at the Anderson Civic Center. Boating Safety 4 The meeting will start at 7:00 PM and run for approximately two hours. Request for email 4 The purpose of this meeting is to provide our members, guests and friends of the lake: Addresses • The ability to hear some very interesting and important speakers relating to Hartwell Lake and the Meet the Directors 5 Savannah River Basin • An update of the activities the LHA team has been working for the past year, Safety Alert! for PDFs 6 • The opportunity to meet your officers and members of the Board of Directors, ask questions of News From The Corps 7 them and all speakers and, Lake Level Data 7 • An opportunity to win one of the great door prizes. This year’s keynote speaker is Colonel Mark S. Held, District Commander, Savannah District, U.S. -

Hartwell Lake News Is FREE! PAID Monroe, GA a Direct Mail out to Lake Front Property Owner on Lake Hartwell and Permit No
Prsrt Std US Postage Hartwell Lake News is FREE! PAID www.hartwelllakenews.com Monroe, GA A direct mail out to lake front property owner on Lake Hartwell and Permit No. 15 is distributed to over 200 locations around the lake covering two states and six counties. Like us on Facebook www.hartwelllakeproperties.com • Hartwell Lake Properties • 1-800-BUY-LAKE Volume 15, Number 4 • December 27 – April 5, 2014 SERVING SC AND GA: ANDERSON, CLEMSON, TOWNVILLE, FAIR PLAY, SENECA, HARTWELL, LAVONIA AND TOCCOA INSIDE Corps to Reduce Visitor Services FEATURED HOME Page 16 Next Year Due to Declining Federal Recreation Funds SAVANNAH, Ga. – Due to significant communities while achieving the projected budget reductions in fiscal year necessary cost reductions. 2014, the U.S. Army Corps of Engineers “We considered altern-atives Savannah District will reduce park to maintaining park operations and 4 Partain Dr. Looking for a Lake Hartwell operations and visitor services at lakes acceptable visitor services within retreat or full time home? This is it! A completely Hartwell and J. Strom Thurmond during the funding limitations, such as complete furnished 3 BD/2BA home located just off Bouy 2014 recreation season. park closures, partial closures, seasonal S21 of the Savannah Main channel of the lake. The Corps will close one campground reductions, and reduced visitor services,” Deep water location with a double deck dock in place & includes a boat lift for your boat. The and five day use areas on Hartwell Lake, and said Peggy O’Bryan, chief of operations home is situated in a very private setting of 1.41 four campgrounds on Thurmond Lake. -
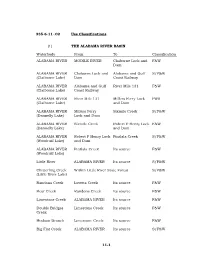
11-1 335-6-11-.02 Use Classifications. (1) the ALABAMA RIVER BASIN Waterbody from to Classification ALABAMA RIVER MOBILE RIVER C
335-6-11-.02 Use Classifications. (1) THE ALABAMA RIVER BASIN Waterbody From To Classification ALABAMA RIVER MOBILE RIVER Claiborne Lock and F&W Dam ALABAMA RIVER Claiborne Lock and Alabama and Gulf S/F&W (Claiborne Lake) Dam Coast Railway ALABAMA RIVER Alabama and Gulf River Mile 131 F&W (Claiborne Lake) Coast Railway ALABAMA RIVER River Mile 131 Millers Ferry Lock PWS (Claiborne Lake) and Dam ALABAMA RIVER Millers Ferry Sixmile Creek S/F&W (Dannelly Lake) Lock and Dam ALABAMA RIVER Sixmile Creek Robert F Henry Lock F&W (Dannelly Lake) and Dam ALABAMA RIVER Robert F Henry Lock Pintlala Creek S/F&W (Woodruff Lake) and Dam ALABAMA RIVER Pintlala Creek Its source F&W (Woodruff Lake) Little River ALABAMA RIVER Its source S/F&W Chitterling Creek Within Little River State Forest S/F&W (Little River Lake) Randons Creek Lovetts Creek Its source F&W Bear Creek Randons Creek Its source F&W Limestone Creek ALABAMA RIVER Its source F&W Double Bridges Limestone Creek Its source F&W Creek Hudson Branch Limestone Creek Its source F&W Big Flat Creek ALABAMA RIVER Its source S/F&W 11-1 Waterbody From To Classification Pursley Creek Claiborne Lake Its source F&W Beaver Creek ALABAMA RIVER Extent of reservoir F&W (Claiborne Lake) Beaver Creek Claiborne Lake Its source F&W Cub Creek Beaver Creek Its source F&W Turkey Creek Beaver Creek Its source F&W Rockwest Creek Claiborne Lake Its source F&W Pine Barren Creek Dannelly Lake Its source S/F&W Chilatchee Creek Dannelly Lake Its source S/F&W Bogue Chitto Creek Dannelly Lake Its source F&W Sand Creek Bogue -

Like No Place on Earth
Like no Place on Earth Heaven’s Landing, the Middle of EVERYWHERE! - Mike Ciochetti EVERYWHERE Heaven’s Landing is a residential fly-in community located in the Blue Ridge Mountains of northeast Georgia. Immediately surrounded by the pristine EVERYTHING serenity of the Chattahoochee National Forest, one could easily get the impression that Heaven’s Landing is located in the middle of nowhere. After all, virtually every photograph that you see of Heaven’s Landing shows a 5,200 The Races foot runway surrounded by colorful mountain wilderness. To the contrary, however, Heaven’s Landing is only 3.5 miles from the City of Clayton, Georgia, and very much in the middle of EVERYWHERE! Fall Sweeps A seven-minute drive from Heaven’s Landing brings you to downtown Clayton, which has the best of just about EVERYTHING! Four of the top 100 rated Where to Find Us chefs in the U.S. operate restaurants in Rabun County to the delight of gourmet foodies. Rabun County was named the “Farm to Table Capital of Georgia!” This is a testament to the great relationships the chefs have with the Circus Arts local farms, following the practices of the finest eateries, and the abundant availability of farm fresh organic meats and produce. If you don’t care to dine out, shoppers will delight in the finest supermarkets, farmer’s markets, Mark Your organic food co-op’s and old fashioned local butcher shops that you will find Calendar ANYWHERE! So Heaven’s Landing has your fine dining needs covered, what is there to do Events to work up an appetite? Once again the answer is EVERYTHING! Let’s start with Lake Burton, a renowned 2,735 acre crystal clear mountain lake where Concierge you can water ski, jet ski, fish, or just cruise on your boat and sunbathe in an atmosphere that is pure nirvana. -

Rule 391-3-6-.03. Water Use Classifications and Water Quality Standards
Presented below are water quality standards that are in effect for Clean Water Act purposes. EPA is posting these standards as a convenience to users and has made a reasonable effort to assure their accuracy. Additionally, EPA has made a reasonable effort to identify parts of the standards that are not approved, disapproved, or are otherwise not in effect for Clean Water Act purposes. Rule 391-3-6-.03. Water Use Classifications and Water Quality Standards ( 1) Purpose. The establishment of water quality standards. (2) W ate r Quality Enhancement: (a) The purposes and intent of the State in establishing Water Quality Standards are to provide enhancement of water quality and prevention of pollution; to protect the public health or welfare in accordance with the public interest for drinking water supplies, conservation of fish, wildlife and other beneficial aquatic life, and agricultural, industrial, recreational, and other reasonable and necessary uses and to maintain and improve the biological integrity of the waters of the State. ( b) The following paragraphs describe the three tiers of the State's waters. (i) Tier 1 - Existing instream water uses and the level of water quality necessary to protect the existing uses shall be maintained and protected. (ii) Tier 2 - Where the quality of the waters exceed levels necessary to support propagation of fish, shellfish, and wildlife and recreation in and on the water, that quality shall be maintained and protected unless the division finds, after full satisfaction of the intergovernmental coordination and public participation provisions of the division's continuing planning process, that allowing lower water quality is necessary to accommodate important economic or social development in the area in which the waters are located. -

Chapter 335-6-11 Water Use Classifications for Interstate and Intrastate Waters
Environmental Management Chapter 335-6-11 DEPARTMENT OF ENVIRONMENTAL MANAGEMENT WATER DIVISION - WATER QUALITY PROGRAM ADMINISTRATIVE CODE CHAPTER 335-6-11 WATER USE CLASSIFICATIONS FOR INTERSTATE AND INTRASTATE WATERS TABLE OF CONTENTS 335-6-11-.01 The Use Classification System 335-6-11-.02 Use Classifications 335-6-11-.01 The Use Classification System. (1) Use classifications utilized by the State of Alabama are as follows: Outstanding Alabama Water ................... OAW Public Water Supply ......................... PWS Swimming and Other Whole Body Shellfish Harvesting ........................ SH Fish and Wildlife ........................... F&W Limited Warmwater Fishery ................... LWF Agricultural and Industrial Water Supply ................................ A&I (2) Use classifications apply water quality criteria adopted for particular uses based on existing utilization, uses reasonably expected in the future, and those uses not now possible because of correctable pollution but which could be made if the effects of pollution were controlled or eliminated. Of necessity, the assignment of use classifications must take into consideration the physical capability of waters to meet certain uses. (3) Those use classifications presently included in the standards are reviewed informally by the Department's staff as the need arises, and the entire standards package, to include the use classifications, receives a formal review at least once every three years. Efforts currently underway through local 201 planning projects will provide additional technical data on certain waterbodies in the State, information on treatment alternatives, and applicability of various management techniques, which, when available, will hopefully lead to new decisions regarding use classifications. Of particular interest are those segments which are currently classified for any usage which has an associated Supp. -

Rights of Way Endangered Species Survey for the Northeast Region of Georgia
RIGHTS OF WAY ENDANGERED SPECIES SURVEY FOR THE NORTHEAST REGION OF GEORGIA. The Northeast Region ofGeorgia includes 24 counties: Banks, Barrows, Clarke, Dawson, Elbert, Fannin, Forsyth, Franklin, Greene, Habersham, Hart, Hall, Jackson, Lumpkin, Madison, Morgan, Oconee, Oglethorpe, Rabun, Stephens, Towns, Union, Walton and White. Based on The Georgia Natural Heritage Inventory records, the following plants and plant community locations were searched for on Georgia Power Company rights ofways: Georgia aster - Aster georgianus Dwarfstonecrop - Sedum pusillum Indian olive - Nestronia umbellulua Silky bindweed - Calystegia catesbiana ssp. sericata Smooth purple coneflower - Echinacea laevigata (Federal Endangered) Ginseng - Panax quinquefolius Pipewort - Eriocaulon koemickianum American Milkwort - Pilularia Americana Granite outcrop (shrub, bare rock, lichen) communities There are three maintenance concerns. Two are minor concerns in Walton county with granite outcrops communities. (See Walton county summary) and one in Stephens county with smooth purnle coneflower (See Stephens county summary). Discussion and recommendations: A few ofthe Northeast Region counties are located in the mountains and foothill region ofGeorgia, but most are in the Piedmont. Much of the Georgia mountain region is owned and managed by the United States Forest Service. Georgia Power has large holdings in this area as well and has been involved with protected species (persistent trillium Trillium persistents and the green salamander aneides aeneus) on company lands at Tallulah Gorge, but these species locations are not close to Georgia Power rights ofways so there are no concerns for right ofway maintenance. The majority oflocations in this region, are within the Piedmont province. Due to a long history ofearly European use (and misuse) for settlement, agriculture and logging, the original Piedmont plant communities ofGeorgia have been altered drastically over the past 2 centuries. -

The Savannah River System L STEVENS CR
The upper reaches of the Bald Eagle river cut through Tallulah Gorge. LAKE TOXAWAY MIDDLE FORK The Seneca and Tugaloo Rivers come together near Hartwell, Georgia CASHIERS SAPPHIRE 0AKLAND TOXAWAY R. to form the Savannah River. From that point, the Savannah flows 300 GRIMSHAWES miles southeasterly to the Atlantic Ocean. The Watershed ROCK BOTTOM A ridge of high ground borders Fly fishermen catch trout on the every river system. This ridge Chattooga and Tallulah Rivers, COLLECTING encloses what is called a EASTATOE CR. SATOLAH tributaries of the Savannah in SYSTEM watershed. Beyond the ridge, LAKE Northeast Georgia. all water flows into another river RABUN BALD SUNSET JOCASSEE JOCASSEE system. Just as water in a bowl flows downward to a common MOUNTAIN CITY destination, all rivers, creeks, KEOWEE RIVER streams, ponds, lakes, wetlands SALEM and other types of water bodies TALLULAH R. CLAYTON PICKENS TAMASSEE in a watershed drain into the MOUNTAIN REST WOLF CR. river system. A watershed creates LAKE BURTON TIGER STEKOA CR. a natural community where CHATTOOGA RIVER ARIAIL every living thing has something WHETSTONE TRANSPORTING WILEY EASLEY SYSTEM in common – the source and SEED LAKEMONT SIX MILE LAKE GOLDEN CR. final disposition of their water. LAKE RABUN LONG CREEK LIBERTY CATEECHEE TALLULAH CHAUGA R. WALHALLA LAKE Tributary Network FALLS KEOWEE NORRIS One of the most surprising characteristics TUGALOO WEST UNION SIXMILE CR. DISPERSING LAKE of a river system is the intricate tributary SYSTEM COURTENAY NEWRY CENTRALEIGHTEENMILE CR. network that makes up the collecting YONAH TWELVEMILE CR. system. This detail does not show the TURNERVILLE LAKE RICHLAND UTICA A River System entire network, only a tiny portion of it. -

GEORGIA TROUT FISHING REGULATIONS Trout Season Trout
GEORGIA TROUT FISHING REGULATIONS Trout Season All designated trout waters are now open year round. Trout Fishing Hours • Fishing 24 hours a day is allowed on all trout streams and all impoundments on trout streams except those in the next paragraph. • Fishing hours on Dockery Lake, Rock Creek Lake, the Chattahoochee River from Buford Dam to Peachtree Creek, the Conasauga River watershed upstream of the Georgia-Tennessee state line and Smith Creek downstream of Unicoi dam are 30 minutes before sunrise until 30 minutes after sunset. Night fishing is not allowed. Trout Fishing Rules • Trout anglers are restricted to the use of one pole and line which must be hand held. No other type of gear may be used in trout streams. • It is unlawful to use live fish for bait in trout streams. Seining bait-fish is not allowed in any trout stream. Impoundments On Trout Streams Anglers can: • Fish for fish species other than trout without a trout license on Dockery and Rock Creek lakes. • Fish at night, except on Dockery and Rock Creek lakes. See Trout Fishing Hours for details. Impoundment notes: • If you fish for or possess trout, you must possess a trout license. If you catch a trout and do not possess a trout license you must release the trout immediately. • State park visitors are not required to have a trout license to fish in the impounded waters of the Park. However, those visitors wishing to harvest trout will need to have a trout license in their possession. Delayed Harvest Streams Anglers fishing delayed harvest streams must release all trout immediately and use and possess only artificial lures with one single hook per lure from Nov. -
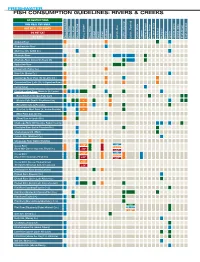
Fish Consumption Guidelines: Rivers & Creeks
FRESHWATER FISH CONSUMPTION GUIDELINES: RIVERS & CREEKS NO RESTRICTIONS ONE MEAL PER WEEK ONE MEAL PER MONTH DO NOT EAT NO DATA Bass, LargemouthBass, Other Bass, Shoal Bass, Spotted Bass, Striped Bass, White Bass, Bluegill Bowfin Buffalo Bullhead Carp Catfish, Blue Catfish, Channel Catfish,Flathead Catfish, White Crappie StripedMullet, Perch, Yellow Chain Pickerel, Redbreast Redhorse Redear Sucker Green Sunfish, Sunfish, Other Brown Trout, Rainbow Trout, Alapaha River Alapahoochee River Allatoona Crk. (Cobb Co.) Altamaha River Altamaha River (below US Route 25) Apalachee River Beaver Crk. (Taylor Co.) Brier Crk. (Burke Co.) Canoochee River (Hwy 192 to Lotts Crk.) Canoochee River (Lotts Crk. to Ogeechee River) Casey Canal Chattahoochee River (Helen to Lk. Lanier) (Buford Dam to Morgan Falls Dam) (Morgan Falls Dam to Peachtree Crk.) * (Peachtree Crk. to Pea Crk.) * (Pea Crk. to West Point Lk., below Franklin) * (West Point dam to I-85) (Oliver Dam to Upatoi Crk.) Chattooga River (NE Georgia, Rabun County) Chestatee River (below Tesnatee Riv.) Chickamauga Crk. (West) Cohulla Crk. (Whitfield Co.) Conasauga River (below Stateline) <18" Coosa River <20" 18 –32" (River Mile Zero to Hwy 100, Floyd Co.) ≥20" >32" <18" Coosa River <20" 18 –32" (Hwy 100 to Stateline, Floyd Co.) ≥20" >32" Coosa River (Coosa, Etowah below <20" Thompson-Weinman dam, Oostanaula) ≥20" Coosawattee River (below Carters) Etowah River (Dawson Co.) Etowah River (above Lake Allatoona) Etowah River (below Lake Allatoona dam) Flint River (Spalding/Fayette Cos.) Flint River (Meriwether/Upson/Pike Cos.) Flint River (Taylor Co.) Flint River (Macon/Dooly/Worth/Lee Cos.) <16" Flint River (Dougherty/Baker Mitchell Cos.) 16–30" >30" Gum Crk. -

Savannah River Basin Management Plan 2001
Savannah River Basin Management Plan 2001 Georgia Department of Natural Resources Environmental Protection Division Georgia River Basin Management Planning Vision, Mission, and Goals What is the VISION for the Georgia RBMP Approach? Clean water to drink, clean water for aquatic life, and clean water for recreation, in adequate amounts to support all these uses in all river basins in the state of Georgia. What is the RBMP MISSION? To develop and implement a river basin planning program to protect, enhance, and restore the waters of the State of Georgia, that will provide for effective monitoring, allocation, use, regulation, and management of water resources. [Established January 1994 by a joint basin advisory committee workgroup.] What are the GOALS to Guide RBMP? 1) To meet or exceed local, state, and federal laws, rules, and regulations. And be consistent with other applicable plans. 2) To identify existing and future water quality issues, emphasizing nonpoint sources of pollution. 3) To propose water quality improvement practices encouraging local involvement to reduce pollution, and monitor and protect water quality. 4) To involve all interested citizens and appropriate organizations in plan development and implementation. 5) To coordinate with other river plans and regional planning. 6) To facilitate local, state, and federal activities to monitor and protect water quality. 7) To identify existing and potential water availability problems and to coordinate development of alternatives. 8) To provide for education of the general public on matters involving the environment and ecological concerns specific to each river basin. 9) To provide for improving aquatic habitat and exploring the feasibility of re-establishing native species of fish.