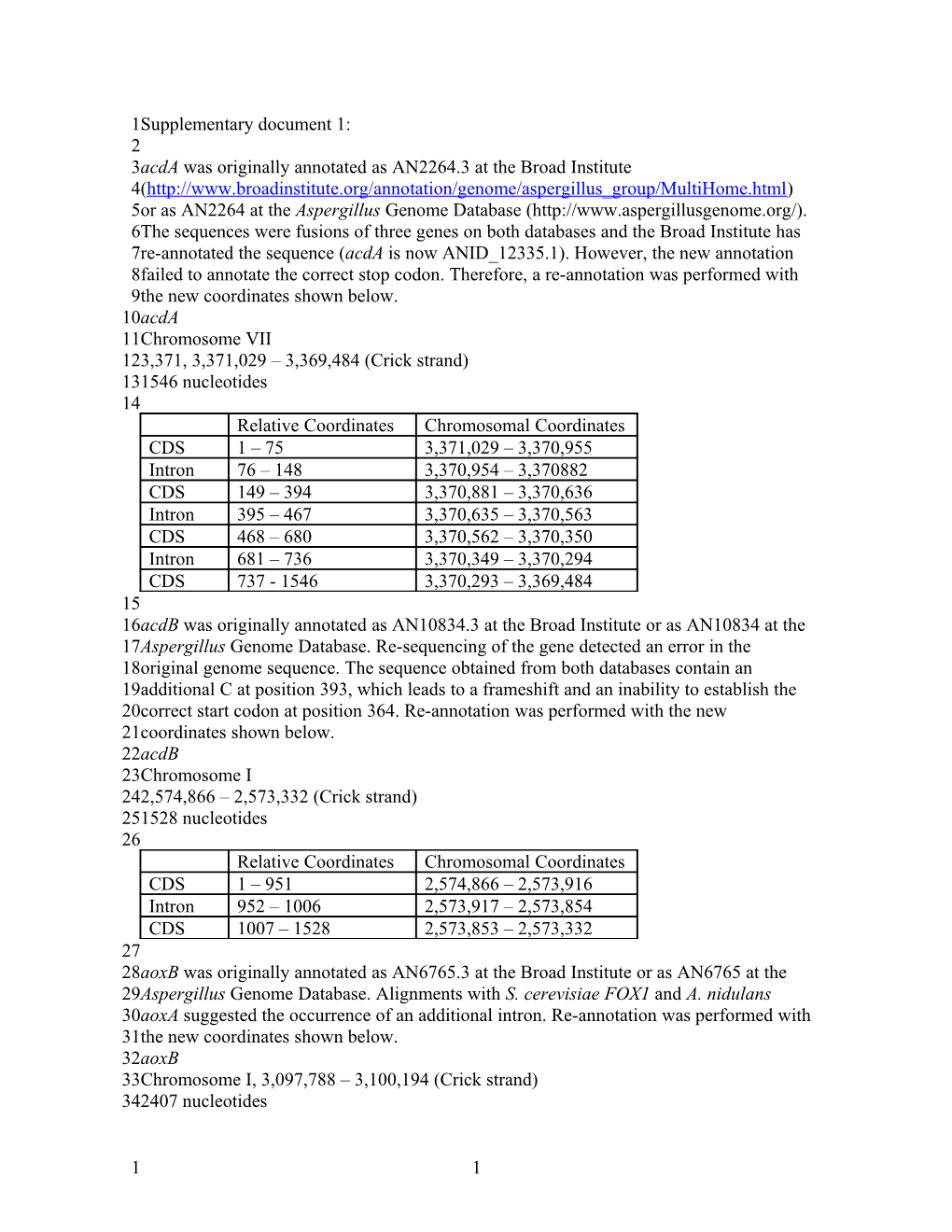
1Supplementary document 1: 2 3acdA was originally annotated as AN2264.3 at the Broad Institute 4(http://www.broadinstitute.org/annotation/genome/aspergillus_group/MultiHome.html) 5or as AN2264 at the Aspergillus Genome Database (http://www.aspergillusgenome.org/). 6The sequences were fusions of three genes on both databases and the Broad Institute has 7re-annotated the sequence (acdA is now ANID_12335.1). However, the new annotation 8failed to annotate the correct stop codon. Therefore, a re-annotation was performed with 9the new coordinates shown below. 10acdA 11Chromosome VII 123,371, 3,371,029 – 3,369,484 (Crick strand) 131546 nucleotides 14 Relative Coordinates Chromosomal Coordinates CDS 1 – 75 3,371,029 – 3,370,955 Intron 76 – 148 3,370,954 – 3,370882 CDS 149 – 394 3,370,881 – 3,370,636 Intron 395 – 467 3,370,635 – 3,370,563 CDS 468 – 680 3,370,562 – 3,370,350 Intron 681 – 736 3,370,349 – 3,370,294 CDS 737 - 1546 3,370,293 – 3,369,484 15 16acdB was originally annotated as AN10834.3 at the Broad Institute or as AN10834 at the 17Aspergillus Genome Database. Re-sequencing of the gene detected an error in the 18original genome sequence. The sequence obtained from both databases contain an 19additional C at position 393, which leads to a frameshift and an inability to establish the 20correct start codon at position 364. Re-annotation was performed with the new 21coordinates shown below. 22acdB 23Chromosome I 242,574,866 – 2,573,332 (Crick strand) 251528 nucleotides 26 Relative Coordinates Chromosomal Coordinates CDS 1 – 951 2,574,866 – 2,573,916 Intron 952 – 1006 2,573,917 – 2,573,854 CDS 1007 – 1528 2,573,853 – 2,573,332 27 28aoxB was originally annotated as AN6765.3 at the Broad Institute or as AN6765 at the 29Aspergillus Genome Database. Alignments with S. cerevisiae FOX1 and A. nidulans 30aoxA suggested the occurrence of an additional intron. Re-annotation was performed with 31the new coordinates shown below. 32aoxB 33Chromosome I, 3,097,788 – 3,100,194 (Crick strand) 342407 nucleotides
1 1 1 Relative Coordinates Chromosomal Coordinates CDS 1 – 776 3,100,194 – 3,099,419 Intron 777 – 875 3,099,418 – 3,099,320 CDS 876 – 1273 3,099,319 – 3,098,922 Intron 1274 – 1315 3,098,921 – 3,098,880 CDS 1316 – 1659 3,098,879 – 3,098,536 Intron 1660 – 1718 3,098,535 – 3,098,477 CDS 1719 – 1800 3,098,476 – 3,098,395 Intron 1801 – 1842 3,098,394 – 3,098,353 CDS 1843 – 1983 3,098,352 – 3,098,212 Intron 1984 – 2042 3,098,211 – 3,098,153 CDS 2043 – 2407 3,098,152 – 3,098,017 Intron 2408 – 2238 3,098,016 – 3,097,957 CDS 2239 – 2407 3,097,956 – 3,097,788 2
1 2 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34Figure S1: Phylogenetic tree of Fox1 and homologues including the putative FA-CoA oxidases AoxA 35 and AoxB 36The phylogenetic tree was produced using ClustalW (accurate) (Thompson et al. 1994), Seqboot and 37Protpars (Felsenstein 1989) in Biomanager by ANGIS (http://www.angis.org.au). The tree was edited using 38TreeView (Page 1998) and is shown as a radial tree. Bootstrap values and scale bar (amino acid 39substitutions/site) are displayed. No out-group has been selected. 40The tree of AoxA and AoxB was produced using alignments of following homologous proteins: A. clavatus 411: ACLA_007790, A. clavatus 2: ACLA_094690 re-annotated; A. flavus 1: AFL2G_00480.2, A. flavus 2: 42AFL2G_06552.2 re-annotated; A. flavus 3: AFL2G_11284.2; A. fumigatus: Afu7g06090 and Afu7g06100 43re-annotated; A. nidulans AoxA: AN6752.3, A. nidulans AoxB: AN6765.3 re-annotated; A. niger: 44e_gw1_5.1444.1; A. oryzae 1: AO090005000479, A. oryzae 2: AO090010000014, A. terreus 1: 45ATEG_04376.1, A. terreus 2: ATEG_06288.1, A. terreus 3: ATEG_06308.1, A. terreus 4: ATEG_09798.1; 46B. cinerea: BC1G_08482.1; C. albicans 1: orf19.1652, C. albicans 2: orf19.1655, C. albicans 3: 47orf19.5723; C. immitis 1: CIMG_01877.3; C. immitis 2: CIMG_10832.3, C. immitis 3: CIMG_11894.3 re- 48annotated; F. graminearum: FGSG_02287.3; N. fischeri: NFIA_027340; S. cerevisiae Fox1: YGL205W; Y. 49lipolytica 1: YALI0C23859, Y. lipolytica 2: YALI0D24750, Y. lipolytica 3: YALI0E06567, Y. lipolytica 4: 50YALI0E27654, Y. lipolytica 5: YALI0E32835, Y. lipolytica 6: YALI0E10857.
1 3 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25Figure S2: Alignment of N. crassa NCU01181.3 and the A. nidulans putative FA-CoA dehydrogenases 26AcdA and AcdB 27The amino acid sequence of N. crassa NCU01181.3 and the re-annotated protein sequences of A. nidulans 28AcdA (AN2264.3) and AcdB (AN10834.3) have been aligned using the programs ClustalW (accurate) 29(Thompson et al. 1994) and Boxshade in Biomanager (http://www.angis.org.au). Identical amino acids are 30shaded in black, similar amino acids in grey. The acyl-CoA dehydrogenase middle domain (first box) and 31C-terminal domain (second box) are marked. 32 33
1 4 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15Figure S3: Localisation of the GFP-AcdA and GFP-AcdB 16Spore suspensions of strains expressing the GFP fusion proteins in a wild-type (WT) background, 17MH11914 (GFP-AcdA) and MH11915 (GFP-AcdB), or in pexE background, MH11920 (GFP-AcdA) and 18MH11921 (GFP-AcdB) were inoculated in supplemented minimal media and incubated overnight at 24ºC. 19Microscope slides were prepared, viewed and photographed. Hyphae were visualised by DIC and GFP 20expression was detected as described in Material and Methods. The magnification is 125x; the scale bar 21represents 20µm. Each construct is listed above to the appropriate panel of photos. 22
1 5 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36Figure S4: Occurrence of the CCGAGG sequence in the promoters of acdA and acdB and induction 37of acdA(p)lacZ and acdB(p)lacZ by different carbon sources 381kb of the promoter (p) of acdA (a) and acdB (b) (-1000 to –1) is shown as a schematic overview in 39intervals of 100bp. CCGAGG sequences are represented by triangles. The numbers above the triangles 40indicate location of the CCGAGG sequence. 41ß-galactosidase assays of acdA(p)lacZ (a) and acdB(p)lacZ (b) in different wild-type, farA∆, farB∆, scfA∆ 42and farA∆farB∆ background were performed. All strains were grown in supplemented glucose-minimal 43media for 16h at 37ºC. Mycelia were washed with and transferred to supplemented media containing either 441% glucose, 50mM proline, 10mM butyrate or 2.5mM oleate as sole carbon source for an additional 4h. 45Standard error was calculated from three independent experiments. All assays are recorded in units per 46minute per mg protein, but the scales differ between the two figures. The strains used for acdA(p)lacZ were 47MH11566, MH11648, MH11649, MH1150 and MH12008. For acdB(p)lacZ, the strains used were 48MH12055, MH12129, MH12130, MH12131. 49
1 6