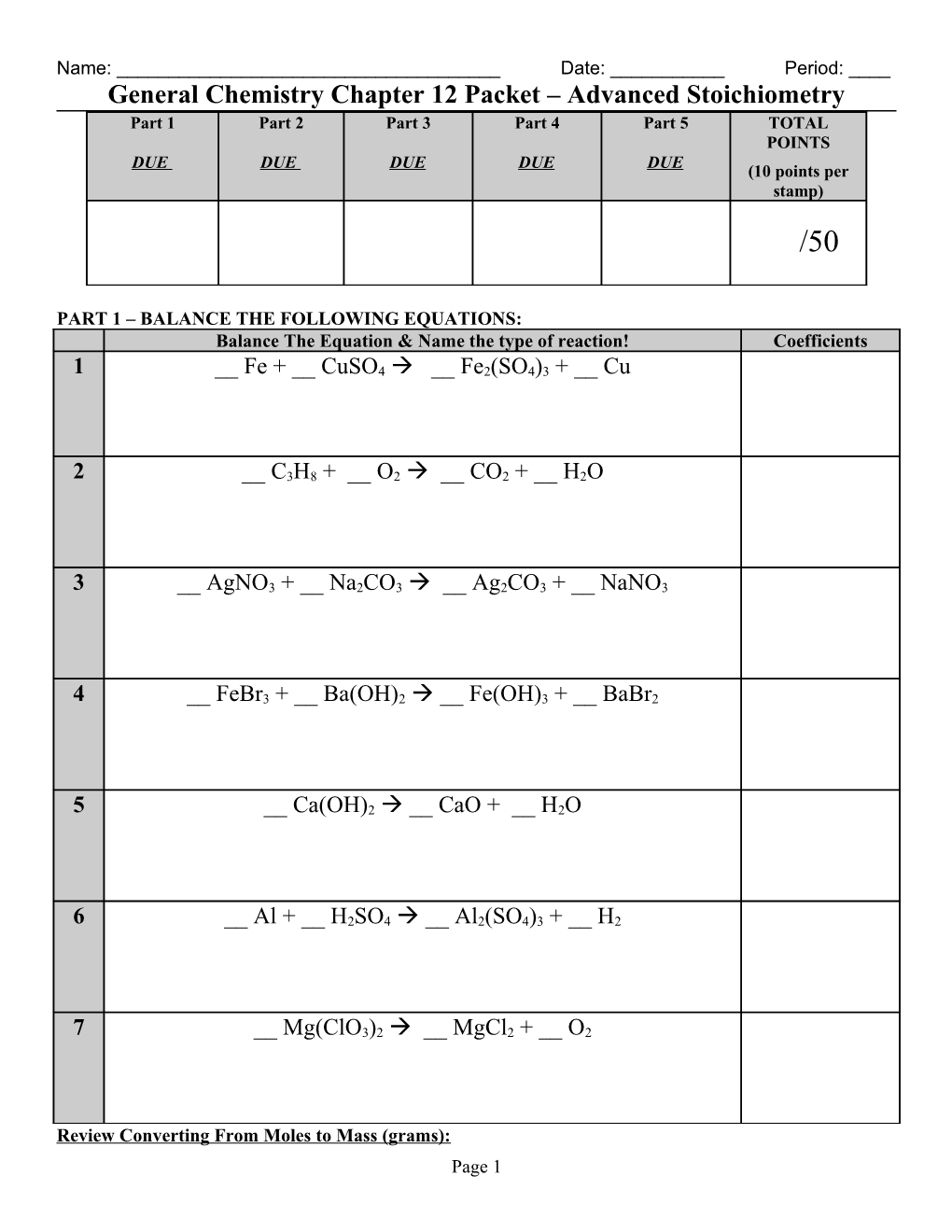
Name: ______Date: ______Period: ____ General Chemistry Chapter 12 Packet – Advanced Stoichiometry Part 1 Part 2 Part 3 Part 4 Part 5 TOTAL POINTS DUE DUE DUE DUE DUE (10 points per stamp) /50
PART 1 – BALANCE THE FOLLOWING EQUATIONS: Balance The Equation & Name the type of reaction! Coefficients
1 __ Fe + __ CuSO4 __ Fe2(SO4)3 + __ Cu
2 __ C3H8 + __ O2 __ CO2 + __ H2O
3 __ AgNO3 + __ Na2CO3 __ Ag2CO3 + __ NaNO3
4 __ FeBr3 + __ Ba(OH)2 __ Fe(OH)3 + __ BaBr2
5 __ Ca(OH)2 __ CaO + __ H2O
6 __ Al + __ H2SO4 __ Al2(SO4)3 + __ H2
7 __ Mg(ClO3)2 __ MgCl2 + __ O2
Review Converting From Moles to Mass (grams): Page 1 1. What is the mass of 3.00moles of NaCl?
2. How many moles are in 27.00g of H2O.
-3 3. What is the mass of 4.52x10 moles of C3H8?
-1 4. Find the number of moles in 3.70x10 grams of Boron.
5. What is the mass of 9.45mol of Al2O3?
6. How many grams are in 2.5mol of Fe(OH)2?
7. Calculate number of moles in 32.0g of CH4.
8. Determine grams in 1.204mole of NH3.
POSSIBLE ANSWERS TO PART 1 -2 2 -1 3.42x10 mol B 1.75x10 g NaCl 1.99x10 g C3H8 2 2 0 2.2x10 g Fe(OH)2 9.64x10 g Al2O3 1.99x10 mol CH4 0 1 1.498x10 mol H2O 2.052x10 g NH3
PART 2 - MOLE RATIOS
Page 2 1. Given this equation: N2 + 3H2 2NH3, write the following molar ratios: N NH a. 2 = b. 3 = H2 H2
2. Given the following equation: 8 H2+ S8 8 H2S, write the following molar ratios:
H2 H2S a. = b. = H2S S8
3. Answer the following questions for this equation: 2H2 + O2 2H2O a. Suppose you had 20 moles of H2, how many moles of H2O could you make?
b. Suppose you had 20 moles of O2, how many moles of H2O could you make?
4. Use this equation: N2 + 3H2 2NH3, for the following problems a. If you used 1 mole of N2, how many moles of NH3 could be produced?
b. If 10 moles of NH3 were produced, how many moles of N2 would be required?
c. If 3.00 moles of H2 were used, how many moles of NH3 would be made?
5. Use the following equation to answer the questions below: N2 + 3H2 2NH3 a. How many moles of NH3 are in 1.75 moles of N2?
b. How many moles of N2 are in 5.23 moles of H2?
c. How many moles of H2 are in 3.02 moles of NH3?
6. Use the following equation to answer the questions below: 2H2 + O2 2H2O a. How many moles of H2 are in 7.10 moles of O2?
b. How many moles of O2 are in 2.27 moles of H2O?
ANSWERS TO PRACTICE #3 1.74 2 2:3 14.2 5 20 2 8:1 1.14 3.5 4.53 40 1:3 8:8 PART 3 - MASS TO MASS CALCULATIONS Page 3 1. 12.0 moles of NaClO3 will produce how many grams of O2? 2 NaClO3 2 NaCl + 3 O2
2. How many grams of Cu are needed to react with 3.50mol of AgNO3? Cu + 2AgNO3 Cu(NO3)2 + 2Ag
3. How many grams of KCl is produced from 2.50moles of K and excess Cl2. 2 K + Cl2 2 KCl
2 4. How many grams of NaOH is produced from 1.2 x 10 moles of Na2O? Na2O + H2O 2 NaOH
2 5. How many grams of Na2O are required to make 1.60 x 10 g of NaOH? Na2O + H2O 2 NaOH
6. What mass of iron is needed to react with 16.0 grams of sulfur (S8)? 8 Fe + S8 8 FeS
7. How many grams NaCl are produced when 80.0g of O2 are produced? 2 NaClO3 2 NaCl + 3 O2
8. If 89.5 moles of Ag were produced, how many grams of Cu reacted? Cu + 2AgNO3 Cu(NO3)2 + 2Ag
9. The average human requires 120.0 grams of glucose (C6H12O6) per day. How many grams of CO2 are required for this amount of glucose? 6 CO2 + 6 H2O C6H12O6 + 6 O2
10. What is the mass of magnesium oxide (MgO) when 10.00 grams of magnesium (Mg) burn in an excess of oxygen, as shown in the following equation? 2Mg + O2 2MgO
POSSIBLE ANSWERS TO PRACTICE #3 16.58 297 0.68 284 124 186 16.58 27.9 576 175.9 1.8 97.4 17.5 9600 PART 4 – MASS TO MASS CALCULATIONS CONTINUED Page 4 11. How many grams of potassium nitrate (KNO3) are required to produce 5.00 g of potassium nitrite (KNO2) according to the following equation? 2KNO3 2KNO2 + O2
12. How many grams of MgCl2 are produced when 8.2 grams of Mg react with an excess of HCl according to the following unbalanced chemical equation: Mg (s) + HCl (aq) MgCl2 (aq) + H2 (g)
13. Determine the mass of lithium hydroxide produced when 0.38 grams of lithium nitride reacts with water according to the following unbalanced chemical equation: Li3N (s) + H2O (l) NH3 (g) + LiOH (aq)
14. What mass of sodium chloride is produced when chlorine gas reacts with 0.29 grams of sodium iodide according to the following unbalanced chemical equation: Cl2 + NaI NaCl + I2
15. Determine the mass of carbon dioxide produced when 0.85 grams of butane (C4H10) reacts with oxygen according to the following unbalanced chemical equation: C4H10 (l) + O2 (g) CO2 (g) + H2O (g)
16. Determine the mass of antimony produced when 0.46 grams of antimony (III) oxide reacts with carbon according to the following unbalanced equation: Sb2O3 (s) + C (s) Sb (s) + CO (g)
POSSIBLE ANSWERS TO PRACTICE #4 0.11 0.38 0.68 2.6 0.78 31 5.94 2760 529 1.8 43.9 17.5 4.77 155 26.4 PART 5 - VOLUME TO VOLUME CALCULATIONS 1. Convert 2.37L of H2 gas at STP to liters of NH3 using the following equation: 3H2 + N2 2NH3
Page 5 2. How many liters of nitrogen dioxide gas are produced when 34L of oxygen react with nitrogen monoxide at STP? 2NO + O2 2NO2
3. How many liters of O2 gas at STP are required to burn 3.86L of carbon monoxide? 2CO + O2 2CO2
4. How many liters of PH3 are formed when 0.42L of hydrogen gas at STP react with phosphorus? P4 + 6H2 4PH3
5. How many liters of NO gas at STP are produced when 1.40L of oxygen reacts with ammonia? 4NH3 + 5O2 4NO + 6H2O
6. How many liters of O2 gas at STP are needed to produce 20.4L of SO3? 2SO2 + O2 2SO3
7. Calculate the volume of sulfur dioxide gas at STP produced when 27.9L of O2 reacts with carbon disulfide. CS2 + 3O2 CO2 + 2SO2
8. How many liters of carbon dioxide are produced when 0.38L of SO2 gas at STP is formed? CS2 + 3O2 CO2 + 2SO2
9. How many liters of NO2 gas at STP are produced if 6.5L of O2 gas reacts? 2NO + O2 2NO2
10. How many liters of NH3 gas at STP are produced when 89.2L of oxygen reacts with ammonia? 4NH3 + 5O2 4NO + 6H2O
ANSWERS TO PRACTICE #5 0.28 1.93 18.6 1.12 10.2 0.19 13 71.4 1.58 68
Page 6