Chapter 12 Gases
Pressure/Temperature Conversions
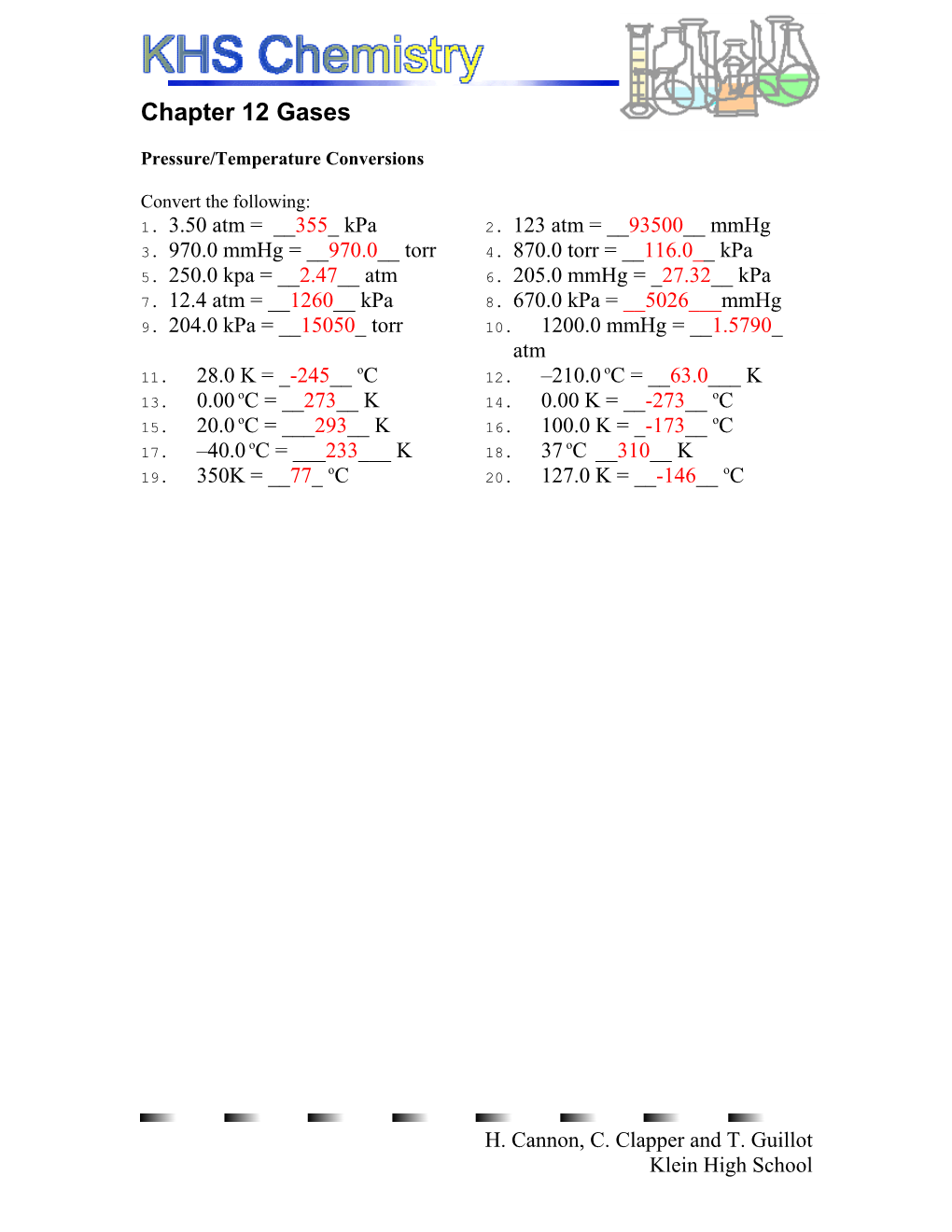
Convert the following: 1. 3.50 atm = __355_ kPa 2. 123 atm = __93500__ mmHg 3. 970.0 mmHg = __970.0__ torr 4. 870.0 torr = __116.0__ kPa 5. 250.0 kpa = __2.47__ atm 6. 205.0 mmHg = _27.32__ kPa 7. 12.4 atm = __1260__ kPa 8. 670.0 kPa = __5026___mmHg 9. 204.0 kPa = __15050_ torr 10. 1200.0 mmHg = __1.5790_ atm o o 11. 28.0 K = _-245__ C 12. –210.0 C = __63.0___ K o o 13. 0.00 C = __273__ K 14. 0.00 K = __-273__ C o o 15. 20.0 C = ___293__ K 16. 100.0 K = _-173__ C o o 17. –40.0 C = ___233___ K 18. 37 C __310__ K o o 19. 350K = __77_ C 20. 127.0 K = __-146__ C
H. Cannon, C. Clapper and T. Guillot Klein High School Gases
Boyle/Charles/Gay-Lussac Exercise 1. What is the pressure of 25.0 ml of a gas if its original volume was 36.0 ml at 810.0 mm of Hg? 810.0 mmHg/ 36.0 ml 1170 mmHg 1 / 25.0 ml 2. A gas collected at 4200.0 mm of Hg has a volume of 31.0 L. if the volume decreases to 28.0 L, what is the pressure of the gas? 4650 mmHg
3. A sample of gas is heated at constant pressure from 1.00oC to 4.00oC. Will the volume increase to four times its original volume? no
4. Calculate the final volume at 71.0oC of a 4.01 L sample of gas that is originally at 23.0oC, assuming that the pressure does not change. 4.65L
5. A light contains neon at a pressure of 2.8 atm with a temperature of 23.0oC. If the light is turned on and its temperature rises to 50.0oC, what is the pressure of the neon?
3.1 atm 6. A cylinder of oxygen has a pressure of 800.0 mm of Hg and a temperature of 21.0oC. To increase the pressure to 239.0 kPa, to what must you change the temperature? 663 K
7. Calculate the final pressure of a sample of gas that is expanded to 1.73 L at constant temperature from 1.50 L at 750.0 torr. 865.0 torr
8. A gas is held in a 100.0L tank at an unknown pressure. When the gas is released into another tank and held at 1.00 atm of pressure, the volume of the gas is 2,500.0L. What was the pressure of the gas in its original tank? 25.0L
9. A sample of gas maintained at a constant pressure is found to have a volume of 3.5 L at 90.0oC. If the system is heated to 100.0oC, what is the resulting volume? 3.5L/ 373K 1 / 363K 3.60L
10. A man heats a balloon in the oven (why, I do not know). If the balloon initially has a volume of 0.400 liters and a temperature of 20.0o C, what will the volume of the balloon be after he heats it to a temperature of 250.0o C? .714 L
11. A cylinder of oxygen has a pressure of 800.0 mm of Hg and a temperature of 21.0oC. To increase the pressure to 239.0 kPa, to what must you change the temperature? 659K
2 HC/CC/TG KHS Gases
12. A 2.00 L sample of gas has a pressure of 1.00 atm. Calculate the volume after its pressure is increased to 1010 torr at constant temperature. 1.51L
13. Calculate the volume of a sample of gas that is initially at 1.00 atm if its volume is increased to 150.0 ml as its pressure is changed to 755 torr at constant temperature. 150.00 ml/ 755 torr 149 ml 1 / 760 torr 14. Calculate the initial volume at 344 K of a sample of gas that is changed to 2.93 L by cooling to 286 K at constant pressure. 2.93L / 344K 3.52 L 1 / 286 K
15. The piston in my car allows it to run. If I want the gas in the piston cylinder to expand from 1.00 liter to 2.50 liters, how hot do I have to make it? Assume the initial temperature is 300.0°K. 7.50 x 102 K
16. For the piston in problem 15, what temperature do I have to make the piston if I want the volume to be 0.280 liters? 84.0 K
17. For the piston in problems 15 and 16, what will be the volume of the piston be if I increase the temperature to 1000.0K?
3.33 L 18. A soda bottle is flexible enough that the volume of the bottle can change even without opening it. If you have an empty soda bottle (volume of 2.00 liters) at room temperature (25.00°C), what will the new volume be if you put it in your freezer (-4.00C)?
1.81L 19. A container is filled with a gas at 290.0 K with a pressure of 4.60 atm. If the container is heated to 405.0 K, what is the new pressure of the gas? 4.60 atm/ 405.0 K 6.42 atm 1 / 290.0 K
20. Given a gas with a pressure of 209 kPa at 18.0oC, what would the pressure change to if the temperature changes to 36.0oC?
222 kPa
3 HC/CC/TG KHS Gases
The Combined Gas Law - 1. Calculate the final pressure of a gas that is expanded from 1.50 L at 355 K and 750.0 torr to 1.73 L at 390.0 K. 750.0 torr/ 1.50 L/ 390.0 K = 714 torr 1 / 355 K / 1.73 L
2. Calculate the volume of a sample of gas originally occupying 505 ml at 750.0 torr and 25.0oC after its temperature and pressure are changed to 50.0oC and 1.21 atm. 446 ml
3. Calculate the original volume of a sample of gas that is at 750.0 torr and 25.0oC before its volume, temperature, and pressure are changed to 505 ml, 50.0oC, and 1.21 atm. 571 ml
4. A 4.00 L sample of gas originally at standard temperature and pressure is changed to 2.95 L at 2,020 torr. Calculate its final temperature in degrees Celsius. 535K 262 degress C 5. Calculate the volume at standard temperature and pressure of a sample of gas that has a volume of 755 ml at 30.0oC and 770.0 torr. 689 ml
6. Given 40.0 ml of nitrogen at 25.0oC and 750.0 mm pressure, calculate its volume at 150.0oC and 650.0 mm pressure. 65.5 ml
o 7. Give 120.0 ml of CO2 at 30.0 C and 800.0 mm pressure, what volume would it occupy at standard conditions?
114 ml
8. Calculate the pressure required to compress 2.00 L of gas at 1.20 atm of pressure and 24.0oC into a 0.25 L container at a temperature of -58.0oC? 6.95 atm
9. A gas occupies 5.80 L at STP. To put the gas into a smaller 0.750 L container at 4.00 atm of pressure, what must the temperature be changed to?
141 K
10. A 980.0 ml balloon at 300.0 K has a pressure of 980.0 mm Hg. If you put the balloon into a refrigerator and the pressure goes down to 75.0 kPa at a volume of 240.0 ml, what is the temperature inside the refrigerator?
4 HC/CC/TG KHS Gases
42.2K
11. If I initially have a gas at a pressure of 12.0 atm, a volume of 23.0 L, and a temperature of 200.0 K, and I raise the pressure to 14.0 atm and increase the volume to 27.0 L, what is the new temperature of the gas? 274K
12. A gas that has a volume of 28.0 L, a temperature of 45.0oC, and an unknown pressure has its volume increased to 35.0 L and its temperature decreased to 35.0oC. If I measure the pressure after the change to be 2.00 atm, what was the original pressure? 2.58 atm 13. Calculate the pressure required to compress 32.0 L of a gas at 6.80 atm pressure and 1500.0oC into a container of 1.5 L capacity at a temperature of 900.0oC. 96 atm
14. Given 60.0 ml of gas at 330.0 K and 720.0 mm of pressure, calculate its volume at 8000.0 K and 0.750 atm of pressure. 1837 ml
15. Given 88.0 L of carbon dioxide at 2700.0 K and 800.0 mm of pressure, what is the temperature of the gas if its volume changes to 50.0 liters at 2.00 atm. 2910 K
16. An automobile tire has a pressure of 210.0 kPa at 20.0oC. What will be the tire pressure after driving, if the tire temperature rises to 35.0oC? 199 kPa
17. A tank for compressed gas has a maximum safe pressure limit of 825 kPa. The pressure gauge reads 388 kPa when the temperature is 24.0 oC. What is the highest temperature the tank can withstand safely?
632 K 18. Maintaining constant pressure, the volume of a gas is increased from 15.0 L to 30.0 L by heating it. If the original temperature was 20.0oC, what is the new temperature (in degrees Celsius)? 586 K
19. In your car's engine, a cylinder has a volume of 420.0 cm3 at the time fuel is injected into it. The gas has a temperature of 32.0oC and a pressure of 92.0 kPa. At the time of firing, the cylinder volume has changed to 49.4 cm3 and the gas is at a temperature of 400.0oC. What is the pressure of the gas at that time? 1726 kPa 20. A. balloon will burst at a volume of 2.00 dm3. If the gas in a partially filled balloon occupies 0.750 dm3 at a temperature of 21.0oC and a pressure of 99.0 kPa,
5 HC/CC/TG KHS Gases
what is the temperature at which it will burst if the pressure is 101 kPa? 8.00 x 102 K
Dalton’s Law of Partial Pressures
1. A mixture of oxygen and nitrogen contains oxygen at a pressure of 200.0 torr and nitrogen at a pressure of 550.0 torr. What is the pressure of the system? 200.0 torr + 550.0 torr = 750.0 torr 2. A mixture of oxygen and nitrogen has a barometric pressure of 1.02 atm. If the pressure of the oxygen is 0.200 atm, what is the pressure of the nitrogen? 0.820 atm 3. A gaseous mixture contains 0.520 moles of H2, 0.233 mole of N2, and 0.941 mole of Ne. The total pressure of the mixture is 1.33 atm. What is the partial pressure of each gas? 0.520 moles + 0.233 moles + 0.941 moles = 1.694 total moles 0.520 moles H2 X 1.33 atm = .408 atm = PH2 .183 atm = PNe .739atm = PN2 1.694 moles total 4. Calculate the total number of moles in a 4.00 L sample of gas at 300.0 K, containing O2 at 0.605 atm and N2 at 0.220 atm. Also calculate the number of moles of O2 present. (0.825 atm)(4.00L) = n (00.0821 atmL/molK)(300.0K total moles = 0.134 moles .605 atmO2 X .134 moles total = 0.0980 moles O2 .825 atm total 5. Oxygen gas is collected over water at a barometric pressure of 775 torr and 25.0oC. What is the pressure of the water vapor? What is the pressure of the oxygen gas? 3.2 kPa is pressure of water vapor 103.3 kPa – 3.2 kPa = 100.1 kPa pressure of the oxygen 6. Oxygen gas is standing over water at a total pressure 770.0 torr. The partial pressure of the oxygen is found to be 750.0 torr. Determine the temperature of the system. 770.0 torr – 750.0 torr = 20.0 torr (pressure of water vapor) 20.0 torr/ 101.3 kPa = 2.667 kPa 1 / 760 torr vapor pressure of water at 22C is 2.6, pressure is 2.8 at 23C temp is approximately 22.5C 7. Calculate the partial pressure in millimeters of mercury exerted by the four main gases in air at 760.0 mm Hg: nitrogen, oxygen, argon, and carbon dioxide. Their abundance by volume is 78.08%, 20.95%, 0.934%, and 0.035%, respectively. N2 = 593.4 mmHg; O2 = 159.2 mmHg; Ar = 7.098 mmHg; CO2 = .266 mmHg o 8. What volume will 2.00 g of O2 occupy when collected over water at 25.0 C and 1.00 atm barometric pressure. 1.58L (101.3kPa-3.2kPa)(X) = (2.00g/32.00g/mole)(8,31kPaL/molK)(298K) 9. A student has stored 100.0 ml of neon gas over water on a day when the temperature is 27.0oC. If the barometer in the room reads 743.3 mm Hg, what is the pressure of the neon gas in its container? 716.3 mmHg
10. Determine the partial pressure of oxygen collected by water displacement if the water temperature is 20.0oC and the total pressure of the gases in the collection bottle is
6 HC/CC/TG KHS Gases
730.0 torr. 712.7 mmHg
Vapor Pressure of Water
Temperature Pressure Temperature Pressure C kPa C kPa 0 0.6 25 3.2 3 0.8 26 3.4 5 0.9 27 3.6 8 1.1 28 3.8 10 1.2 29 4.0 12 1.4 30 4.2 14 1.6 32 4.8 16 1.8 35 5.6 18 2.1 40 7.4 19 2.2 50 12.3 20 2.3 60 19.9 21 2.5 70 31.2 22 2.6 80 47.3 23 2.8 90 70.1 24 3.0 100 101.3
7 HC/CC/TG KHS Gases
Molar Mass Of A Gas
1. Calculate the volume that 2.68 g of oxygen gas occupies at standard temperature and pressure. 2.68g O2/1 molO2/22,4L O2 = 1.88L 1 /32.0g O2/1 mol O2
o 2. Calculate the volume that 3.26 mols of N2 occupies at 0 C and 1.00 atm. 73.0 L
3. What volume will 1.216 g of SO2 gas occupy at STP conditions? .426 L
4. Compute the mass of one liter of ammonia gas at 0oC and a pressure of 101.3 kPa. .759 g
5. Calculate the mass of 4.00 liter of N2O at 273K and a pressure of 760.0 mmHg. 7.86L
6. Calculate the number of liters occupied by 0.245 mole of F2 at STP. 5.49L
7. Calculate the molecular mass of a gas with a density of 1.65 g/l at STP. 36.96 g/mol
8. Calculate the density at STP of ammonia gas. .759 g/L
9. Calculate the density at STP of chlorine gas.
3.165 g/L
8 HC/CC/TG KHS Gases
The Ideal Gas Law
1. Determine the number of moles of gas in a volume of 1.50 L at 355 K and 750.0 mmHg. (750.0mmHg)(1.50L) = n (62.4 mmHGL/molK)(355K) 0.0510 moles = n
o 2. Determine the volume of 0.146 mole of O2 at 35.0 C and 792 mmHg. 3.53L
3. Determine the pressure of 0.0153 mole of CO2 gas with a volume of 1.50 L at 285 K. .239 atm or 181.4 mmHg or 24.18 kPa
4. Determine the number of moles of gas in a volume of 1.33 L at 355 K and 1.12 atm. 0.0510 moles
5. Determine the temperature of a gas if 1.45 moles occupies 10.7 L at 0.965 atm. Is the gas more likely to be Ne or H2O? Explain. 86.74 K Ne (water would be frozen at that temperature)
6. Calculate the volume of 2.00 mole of carbon dioxide gas at 373 K and 1.00 atm. 61.2 L
7. Calculate the volume of 1.50 mole of methane gas at 300.0 K and 1.265 atm. 29.2 L
8. Calculate the volume of 2.00 mole of oxygen gas at 30.0oC and 755 torr. 50.1L
9. Calculate the volume of 50.0 g of oxygen gas at 50.0oC and 380.0 torr. 82.9 L
10. Calculate the volume of 2.00 mole of gas at 100.0oC and 785 torr. 59.3 L
11. Calculate the pressure of 22.0 g of oxygen gas that occupies 16.4 L at 16.0oC. 0.995 atm or 100.76 kPa or 756 mm/hg
12. Calculate the number of moles of oxygen gas in a 3.00 L container at 44.0oC and 768 mmHg. 0.116 moles
9 HC/CC/TG KHS Gases
Ideal Gas Law - Exercise 2 o 1. A 5.00 liter flask, at 25.0 C contains a 0.200 mole of Cl2. What is the pressure in the flask? .979 atm or 99.14 kPa or 744 mmHg 2. What volume will 12.0 g of oxygen occupy at 15.0oC and a pressure of 0.520 atm? 17.1 L 3. What pressure will be exerted by 0.450 mole of a gas at 25.0oC if it is contained in a vessel whose volume is 0.780 L? 14.1 atm or 10730 mmHg or 1430 kPa 4. How many moles of H2 are present in a 2.70 L cylinder if the pressure is 2.10 atm and the temperature is 45.0oC? .217 moles 5. Determine the molecular weight of a gas if 1200.0 ml weigh 1.90 g at STP. 35.49 g/mol 6. The density of a gas is 0.620 g/L at STP. Find the mass of 6.40 L of the gas at a temperature of 21.0oC and a pressure of 762 mm of Hg. .620g/22.4 L=13.888g/mol 1L/ 1 mole (762mmHg)(6.40L) = N (62.4 mmHgL/molK)(294K) = .266 mol .266mol/13.888g = 3.69g 1 / 1 mol 7. The density of a gas is 0.130 g/L at STP. Find the mass of 2.80 L of the gas at a temperature of 18.0oC and 750.0 mm Hg pressure. 0.337 g 8. Calculate the pressure of a gas at 14.0oC if its volume does not change and its original temperature was 6.80oC with a pressure of 1.00 kPa. 1.05 kPa 9. Determine the molecular weight of a gas if 1050.0 ml weighs 1.10 g at STP. 23.5g/mol 10. Determine the volume occupied by 16.0 moles of a gas at 13.0oC and 1.90 kPa. 2.00 x 104 L 11. What is the pressure of 15.0 ml of a gas if its original volume was 26.0 ml at 810.0 mm of Hg (temperature remains constant)? 1404 mmHg 12. A gas collected at 48.0oC has a volume of 31.0 L. If the volume decreased to 29.0 L and pressure remains constant, what is the temperature of the gas (in degrees Celsius)? 3.00 x 102K 13. Given 60.0 ml of a gas at 330.0 K and 720.0 mm of pressure, calculate its volume at 80.0oC and 0.750 atm of pressure. 81.1 ml 14. Calculate the pressure of a gas at 29.0oC if its volume does not change and its original temperature was 26.8oC with a pressure of 2.20 kPa. 2.22 kPa 15. Determine the molecular weight of a gas if 970.0 ml weigh 0.900 g at STP. 20.8 g/mol 16. Determine the volume occupied by 1.30 moles of a gas at 23oC and 1.26 kPa. 2540 L 17. What pressure must be applied to contain 1.216 grams of sulfur dioxide in a 456 ml container at 105 C? 1.34atm (136 kPa 1020mmHg) 18. Compute the number of grams of oxygen that are contained in a 10.5 liter container at 30.0C under 740.0 mmHg of pressure. 132 g 19. How many moles of hydrogen gas are present in a 50.0 L steel cylinder at 10.0 atm of pressure and 27.0 C? 20.3 moles 20. What volume will be occupied by 150.0 grams of chlorine gas at 0.970 atm of pressure and –12.5C? 46.6 L
10 HC/CC/TG KHS Gases
Density of A Gas and Molecular Weight of a Gas
1. The density of a gas is 1.90 g/L at STP. Find the mass of 1.30 L of the gas at a temperature of 8.00oC and 770.0 mm Hg pressure. 1.90g/22.4L = 42.56 g/mol is the molecular mass of the gas 1L / 1 mol (770.0 mmHg)(1.30L) = n (62.4 mmHg)(281K) 0.057 mol/42.56 g = 2.43 g 1 / 1 mole 2. Determine the molecular weight of a gas if 500.0 ml weigh 8.00 g at STP. 8.00g / 22.4L = 358 g/mol .500 L/1 mol
3. The density of a gas is 2.90 g/L at STP. Find the mass of 21.2 L of the gas at a temperature of 28.0oC and 690.0 mm Hg pressure. 52.93 g
4. Determine the molecular weight of a gas if 8.20 x 10-3 g at STP has a volume of 102 ml. 1.80 g/mol
5. A balloon is filled with 1.00 g of a gas at STP. What is the molecular weight of the gas if its volume if 1.10 L? 20.4 g/mol
6. A cylinder contains a gas at 21.0 atm of pressure. The volume of the cylinder is 20.0 L. If the gas has a density of 1.30 g/L, what would its mass be? The temperature of the gas is 23.0oC. 503 g/mol
7. The density of phosphorous triflouride is 3.90 g/cm3. What is the molecular mass of this gas at STP? 87.36 g/mol
8. What is the density of hydrosulfuric acid at 20.0C and 600.0 mmHg of pressure? 1.12 g/L
9. Compute the density of methane (CH4) at 20.0C and 5.00 atm. 3.54 g/L
11 HC/CC/TG KHS Gases
Reactions At Standard Conditions
1. Calculate the number of liters of hydrogen gas at STP that could be produced by the reaction of 6.90 g of magnesium. Mg + 2HCl ---> MgCl2 + H2
6.90g Mg/1 mol Mg/1 mol H2 /22.4 L H2 = 6.36L H2 1 /24.3 g Mg/1 mol Mg/1 mol H2
2. How many grams of H2SO4 must react with Fe to form 5.00 liters of H2 at STP? Fe + H2SO4 ---> FeSO4 + H2 21.88 g H2SO4
3. How many liters of H2F2 will be produced from 37.0 g of fluorine gas at STP? H2 + F2 ---> H2F2
21.81L
4. How many liters of oxygen, measured at STP, must be present to react with 75.0 liters of nitrogen oxide? 2NO + O2 ---> 2NO2
1.50 x 102 L
5. How many liters of NO2 will be produced from 50.0 liters of nitrogen oxide at STP? 2NO + O2 ---> 2NO2 50.0 L
6. How many liters of oxygen will react with 10.0 liters of H2 at STP? 2H2 + O2 ---> 2H2O
5.0 L
12 HC/CC/TG KHS Gases
Reactions at Standard Conditions - Exercise 2
1. If an electric discharge produced 20.0 liters of O3, how many grams of oxygen are required? 3O2 2O3 640 g
2. If 40.0 g of H3PO4 react with magnesium carbonate, calculate the volume of carbon dioxide produced at STP. 2H3PO4 + 3MgCO3 Mg3(PO4)2 + 3CO2 +3H2O
13.7 L
3. Calcium carbide (CaC2) reacts with water to produce calcium hydroxide and C2H2. What volume of the gas at STP could be produced from the reaction of 50.0 g of calcium carbide? CaC2 + 2H2O Ca(OH)2 + C2H2
17.5 L
4. How many grams of sodium hydrogen carbonate must be decomposed to produce 45.0 liters of carbon dioxide? The other products are water and sodium oxide. 2NaHCO3 2CO2 + H2O + Na2O 169 L
5. If 3.20 g of aluminum react with excess hydrogen chloride, how many liters of hydrogen are produced at STP? 2Al +6HCl 2AlCl3 + 3H2
3.99L
6. In a reaction involving carbon monoxide and iron (III) oxide, the products are iron and carbon dioxide. If 84.75 liters of carbon dioxide gas are produced, how many milliliters of carbon monoxide gas are required at STP? 3CO + Fe2O3 2Fe + 3CO2
105.9 L
7. Iron combines with oxygen to form iron (III) oxide. If 450.0 g of iron are used , how many liters, at STP, of oxygen is needed? 4Fe + 3O2 2Fe2O3
135.4 L
8. Ammonia combines with oxygen to form nitrogen monoxide and water. If 850.0 g of ammonia is used, how many liters of nitrogen monoxide are produced at STP? 4NH3 + 5O2 4NO + 6H2O 1120 L
13 HC/CC/TG KHS Gases
9. Dinitrogen trioxide combines with water to form hydrogen nitrite. If 1450 liters of dinitrogen trioxide is used, how many grams of water is needed for the reaction at STP?
N2O3 + H2O 2HNO2 1170 g
10. 500.0 g of aluminum is used in a reaction along with nitrogen. Calculate the number of liters of nitrogen needed to complete the reaction at STP.
2Al + N2 2AlN 208L
11. What volume will 21.7 g of CO2 gas occupy at STP?
11.0 L
12. How many moles of fluorine gas can or will be derived from 7600.0 ml of the gas when at STP? .339 moles
13. C4H10 burns completely to form carbon dioxide and water. If 150.2 liters of butane are used, what volume of oxygen, at STP, must combine with it ?
2C4H10 + 13O2 8CO2 + 10H2O
976.3 L
14. What mass of calcium hydroxide is required to react with 179.2 liters of carbon dioxide to form calcium carbonate and water?
Ca(OH)2 + CO2 CaCO3 + H2O 592g
14 HC/CC/TG KHS Gases
Stoichiometry - Nonstandard Conditions - Bonus
1. Butane, C4H10, burns completely to form carbon dioxide and water. If 150.2 liters of butane are used, what volume of oxygen must combine with it?
2. In the reaction Fe2O3 + 3CO 3CO2 + 3Fe, how many liters of carbon monoxide are needed to form 660.0 liters of carbon dioxide?
3. What weight of calcium hydroxide is required to react with 179.2 liters of carbon dioxide to form calcium carbonate and water?
4. What volume of hydrogen at standard conditions will be produced from the electrolysis of 288 g of water?
5. Aluminum reacts with sodium hydroxide to form sodium aluminate, Na3AlO3, and hydrogen gas. What weight of sodium aluminate is formed in the react which also produces 112 liters of hydrogen at STP?
15 HC/CC/TG KHS Gases
Charles Law Investigation What is Absolute Zero?
Background: Jacques Alexander Cesar Charles, in 1787 studied the effect of temperature change on the behavior of a gas. Charles reasoned that there was a direct relationship between the volume of a gas and the absolute temperature at a constant pressure. He stated that the volume of a fixed quantity of gas at constant pressure increases in a linear fashion with the absolute temperature. In developing a useful tool for predicting the behavior of gases, Charles was aided by Sir William Thompson, a Scottish nobleman whose title was Lord Kelvin. Kelvin, in 1848 proposed the idea of an absolute temperature scale that would eliminate the problems inherent in the current scales when the temperature fell below zero.
Objective: To experimentally verify the value of absolute zero
Determination of Volume Change due to Temperature: 1. Fill two 400-ml beaker half full with tap water. 2. Begin heating the water in one beaker to a temperature that is 10C above room temperature. 3. Fill a thin stem pipette completely with room-temperature water. To make sure the pipette is filled, first draw in as much water as possible. Then, holding the pipette by the bulb with the stem pointing upward, squeeze the bulb slightly to eject any air left in the bulb and stem. Keeping this pressure on the bulb, insert the tip of the stem into the water. Release the pressure on the bulb, and the pipette will fill completely. 4. Dispense the water from the pipette, counting the total number of drops it takes to empty the pipette. (The number should be about 100 drops) 5. Stop heating the hot water beaker when the temperature has risen about 10C above room temperature. 6. Holding the thin stem pipette by the stem, immerse the bulb in the warm water. 7. Hold the pipette in the warm water for a few minutes so that the air in the pipette reaches the temperature of the water. 8. Pinch the stem of the pipette to seal off the bulb. Place the bulb in the room- temperature beaker of water. 9. Still pinching the stem, immerse the entire pipette, including the stem, in the water. Release the stem underwater. A small amount of water should be drawn up into the pipette. This water is equal in volume to the amount of gas lost when the pipette bulb was heated and the air inside it expanded (thus it represents the increase in volume of the air inside the pipette). 10. Remove the pipette from the water bath. Dry the outside of the pipette with a paper towel. Expel the water, counting the number of drops of water that were drawn into the pipette. 11. Add the number of drops (representing the increased volume of the air) to the initial (room-temperature) volume to get the new volume at this temperature.
16 HC/CC/TG KHS Gases
12. Dry the inside of the pipette by drawing in air and then releasing the bulb several times. 13. Repeat this process to record the volume change at five points relatively evenly spaced between 20C and 75C.
Calculation of Absolute Zero: In science, graphing is an important tool used to determine the relationship between two variables. As described by Charles, there is a direct relationship between volume and temperature. We will show this relationship both graphically and mathematically as we extrapolate the data we collected to determine an experimental value for absolute zero. 1. Graph (on paper) your five data points. Draw a straight line through the points and extrapolate below the X-axis until the line intersects the Y-axis. This point will be your absolute zero (the temperature at which the volume becomes 0). 2. Check your value for absolute zero mathematically using a spreadsheet such as Microsoft Excel a. Enter your data into the spreadsheet b. Using the equation for a line, enter calculations to determine the slope of your line c. Using the equation for a line, enter calculations to determine where your line intersects the Y-axis. 1. Use your spreadsheet to graph your data and compare it to your hand drawn graph. 2. Choose the value for absolute zero that you feel is the best answer. 3. Calculate the percent error between your chosen value and actual absolute zero (0 K). 4. Percent error =(actual value–experimental value/actual value) x 100
Notes: 1. Remember when you are working in Excel that you need only refer to the position of the data rather than the absolute numbers, thus the same formula will work for all data points. 2. Remember you guidelines for graphing and use them to verify your procedure a. Dependent (y axis – temperature oC) vs. independent(x axis – number of drops) variable b. Positive vs. negative slope – use to predict if you are doing the graph correctly 3. Include the following with your lab report: a. Hand drawn graph b. Spreadsheet graph c. Spreadsheet printout showing calculations d. Percent error calculation
17 HC/CC/TG KHS Gases
Molar Volume Lab Problem: Using the following reaction, determine the molar volume of hydrogen gas produced. Mg + 2HCl MgCl2 + H2 Procedure: Begin with a few centimeters of magnesium ribbon ( you must determine its mass). Obtain a collecting tube (eudiometer) from you instructor, who will add approximately 5.0 ml of HCl (12M). Fill the tube with water.
Roll the magnesium into a small coil, secured with string, and push the coil into the tube with the thread extending. Invert the tube in a large beaker about half-filled with water. As the higher density HCl falls to the bottom of the tube the reaction will proceed. When the reaction is complete, measure the volume of hydrogen gas produced inside the tube in ml (this will be V1). Record the temperature and barometric pressure in the room. CLEAN UP.
Determine an accurate measurement of the volume of hydrogen produced and convert to STP using the following steps: 1. Correct the barometric pressure for the presence of water vapor 2. Convert the volume to STP Determine the number of moles of magnesium used in the reaction and use the stoichiometry of the reaction to determine the number of moles of hydrogen gas produced.
Use your liters of gas and moles of gas produced to find the volume of one mole of hydrogen gas at STP.
Calculate your percent error, compared to the standard molar volume of a gas.
Mass of Mg g
Volume of gas produced (V1) ml Barometric pressure mmHg Temperature °C
Chapter 12
18 HC/CC/TG KHS