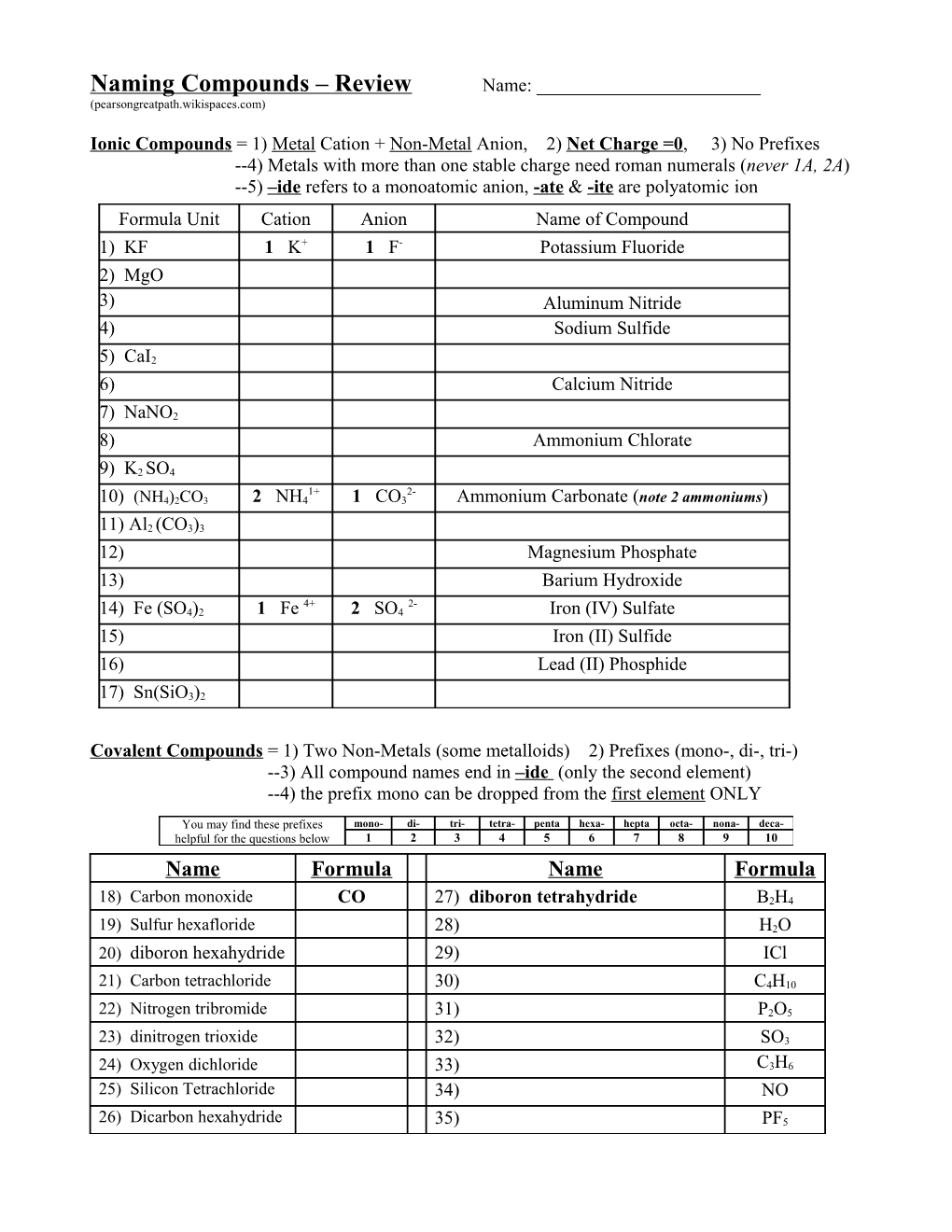
Naming Compounds – Review Name: ______(pearsongreatpath.wikispaces.com)
Ionic Compounds = 1) Metal Cation + Non-Metal Anion, 2) Net Charge =0, 3) No Prefixes --4) Metals with more than one stable charge need roman numerals (never 1A, 2A) --5) –ide refers to a monoatomic anion, -ate & -ite are polyatomic ion Formula Unit Cation Anion Name of Compound 1) KF 1 K+ 1 F- Potassium Fluoride 2) MgO 3) Aluminum Nitride 4) Sodium Sulfide
5) CaI2 6) Calcium Nitride
7) NaNO2 8) Ammonium Chlorate
9) K2 SO4 1+ 2- 10) (NH4)2CO3 2 NH4 1 CO3 Ammonium Carbonate (note 2 ammoniums)
11) Al2 (CO3)3 12) Magnesium Phosphate 13) Barium Hydroxide 4+ 2- 14) Fe (SO4)2 1 Fe 2 SO4 Iron (IV) Sulfate 15) Iron (II) Sulfide 16) Lead (II) Phosphide
17) Sn(SiO3)2
Covalent Compounds = 1) Two Non-Metals (some metalloids) 2) Prefixes (mono-, di-, tri-) --3) All compound names end in –ide (only the second element) --4) the prefix mono can be dropped from the first element ONLY
You may find these prefixes mono- di- tri- tetra- penta hexa- hepta octa- nona- deca- helpful for the questions below 1 2 3 4 5 6 7 8 9 10 Name Formula Name Formula
18) Carbon monoxide CO 27) diboron tetrahydride B2H4
19) Sulfur hexafloride 28) H2O 20) diboron hexahydride 29) ICl
21) Carbon tetrachloride 30) C4H10
22) Nitrogen tribromide 31) P2O5
23) dinitrogen trioxide 32) SO3
24) Oxygen dichloride 33) C3H6 25) Silicon Tetrachloride 34) NO
26) Dicarbon hexahydride 35) PF5 Identify each as either ionic or covalent compounds and provide either the name or formula Covalent Compounds = 1) Two Non-Metals (some metalloids) 2) Prefixes (mono-, di-, tri-) Ionic Compounds = 1) Metal Cation + Non-Metal Anion, 2) Net Charge =0, 3) No Prefixes Name or Formula Ionic or Cation & Anion Name or Formula Covalent
36) nitrogen trihydride Covalent N/A - Covalent NH3 = (Ammonia) 1+ 1- 37) NH4F Ionic 1 NH4 1 F Ammonium Fluoride 38) aluminum hydroxide
39) SnF2 40) carbon tetrahydride 41) iron (III) Nitrate 42) CuS
43) SiO2 44) sulfur dioxide
45) (NH4)2 SO2
46) S2Cl2 47) sulfur dioxide 48) calcium chlorite 49) cobalt(II) hydroxide 50) carbon monoxide
51) CoBr2 52) boron trifluoride
53) Fe2(SO3)3
54) C3H8 55) chlorine monofluoride 56) magnesium nitrate
-Flash Cards – Naming compounds (Ionic, Acidic, and Molecular) http://www.quia.com/jfc/656925.html http://www.quia.com/jg/696905.html http://www.quia.com/jfc/397006.html
-Quiz about compounds http://www.quia.com/jq/19617.html
-Challenge Board (need to discuss polar molecules and like dissolves like) http://www.quia.com/cb/139633.html http://www.quia.com/cb/453451.html - electronegativity
-Rags to Riches http://www.quia.com/rr/131189.html - Chemical bonding (all forms)