Redalyc.Equine Nasopharyngeal Cryptococcoma Due To
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Next-Generation Sequencing for Hypothesis-Free Genomic Detection
Frickmann et al. BMC Microbiology (2019) 19:75 https://doi.org/10.1186/s12866-019-1448-0 RESEARCH ARTICLE Open Access Next-generation sequencing for hypothesis- free genomic detection of invasive tropical infections in poly-microbially contaminated, formalin-fixed, paraffin-embedded tissue samples – a proof-of-principle assessment Hagen Frickmann1,2* , Carsten Künne3, Ralf Matthias Hagen4, Andreas Podbielski2, Jana Normann2, Sven Poppert5,6, Mario Looso3 and Bernd Kreikemeyer2 Abstract Background: The potential of next-generation sequencing (NGS) for hypothesis-free pathogen diagnosis from (poly-)microbially contaminated, formalin-fixed, paraffin embedded tissue samples from patients with invasive fungal infections and amebiasis was investigated. Samples from patients with chromoblastomycosis (n = 3), coccidioidomycosis (n = 2), histoplasmosis (n = 4), histoplasmosis or cryptococcosis with poor histological discriminability (n = 1), mucormycosis (n = 2), mycetoma (n = 3), rhinosporidiosis (n = 2), and invasive Entamoeba histolytica infections (n = 6) were analyzed by NGS (each one Illumina v3 run per sample). To discriminate contamination from putative infections in NGS analysis, mean and standard deviation of the number of specific sequence fragments (paired reads) were determined and compared in all samples examined for the pathogens in question. Results: For matches between NGS results and histological diagnoses, a percentage of species-specific reads greater than the 4th standard deviation above the mean value of all 23 assessed sample materials was required. Potentially etiologically relevant pathogens could be identified by NGS in 5 out of 17 samples of patients with invasive mycoses and in 1 out of 6 samples of patients with amebiasis. Conclusions: The use of NGS for hypothesis-free pathogen diagnosis from contamination-prone formalin- fixed, paraffin-embedded tissue requires further standardization. -

Sporothrix Schenckii: ➢ Thermal Dimorphic
Subcutaneous mycoses ➢1-Mycetoma ➢2-Sporotrichosis ➢3-Chromoblastomycosis ➢4-Rhinosporidiosis ➢5- Lobomycosis ➢6- Entomophthoramycosis Sporotrichosis Rose gardener’s disease Chronic desease Agent Sporothrix schenckii: ➢ Thermal dimorphic ➢ In soil ➢ On decaying vegetation,plants,plant products (hay, straw, sphagnum moss), and a variety of animals (cats) ➢ less than 37° C (hyphal) ➢ 37° C (yeast) ➢ Sporothrix brasiliensis ➢ Sporothrix globosa Epidemiology ➢Worldwide ➢Tropical regions ➢Mexico ➢Brazile ➢France ➢USA Occupational disease: ➢Farmers ➢ ➢Workers ➢Gardeners ➢Florists Predisposing factors: ➢Trauma ➢Inhalation (very rarely) ➢HIV Clinical Syndromes 1-lymphocutaneous 2-Fixed cutaneous 3- Osteoarticular involvement 4-Pulmonary 5-Systemic Primary infection 1-Lymphocutaneous sporotrichosis 2-Fixed cutaneous sporotrichosis: Fixed cutaneous sporotrichosis verrucous-type sporotrichosis localized cutaneous type Paronychia sporotrichosis Osteoarticular involvement Pulmonary sporotrichosis: ➢Alcoholic ➢Pulmonary tuberculosis, diabetes mellitus and steroid ➢A productive cough ➢Low-grade fever ➢Weight loss Systemic sporotrichosis Transmission: ➢Dog bite ➢parrot bite ➢Insects bite ➢Cases of animal-to-human transmission Laboratory Diagnosis: 1-Collection of samples: ➢Drainage from skin lesions ➢Exudates ➢Pus ➢Blood ➢Pulmonary secretions ➢Tissue biopsy specimens 2-Direct examination ➢Gram ➢PAS ➢GMS ➢H & E ❖Yeast Cells ❖Asteroid body: Elongated Buds (“Cigar Body”) Wet Mount BHI Blood 37˚C Yeast with Elongated Daughter Cell Biopsy of subcutaneous tissue -

Boards' Fodder
boards’ fodder Medical Mycology By Adriana Schmidt, MD, and Natalie M. Curcio, MD, MPH. (Updated July 2015*) SUPERFICIAL ORGANISM CLINICAL HISTO/KOH TREATMENT MYCOSES* Pityriasis Malessezia furfur Hypo- or hyper-pigmented Spaghetti & meatballs: Antifungal shampoos and/or versicolor macules short hyphae + yeast PO therapy Tinea nigra Hortaea werneckii (formerly Brown-black non-scaly Branching septate hyphae Topical imidazoles or palmaris Phaeoannellomyces werneckii) macules + budding yeast allylamines Black piedra Piedraia hortae Hard firm black Dark hyphae around concretions acrospores Cut hair off, PO terbinafine, White piedra Trichosporon ovoides or inkin Soft loose white Blastoconidia, imidazoles, or triazoles (formely beigelii) concretions arthroconidia Fluorescent small Microsporum Canis KOH: spores on outside spore ectothrix: M. audouinii of the hair shaft; “Cats And Dogs M. distortum Wood’s lamp --> yellow Sometimes Fight T. schoenleinii fluorescence & Growl” M. ferrugineum+/- gypseum Large spore Trichophyton spp. (T. tonsurans in North America; T. violaceum in KOH: spores within hair Topical antifungals; PO endothrix Europe, Asia, parts of Africa). shaft antifungals for T. manuum, Tinea corporis T. rubrum > T. mentag. Majocchi’s granuloma: T. rubrum capitis, unguium T. pedis Moccasin: T. rubrum, E. floccosum. Interdigital/vesicular: T. mentag T. unguium Distal lateral, proximal and proximal white subungual: T. rubrum. White superficial: T. mentag. HIV: T. rubrum SUBQ MYCOSES** ORGANISM TRANSMISSION CLINICAL HISTO/KOH TREATMENT -

Rhinosporidium Seeberi: a Human Pathogen from a Novel Group of Aquatic Protistan Parasites
Research Rhinosporidium seeberi: A Human Pathogen from a Novel Group of Aquatic Protistan Parasites David N. Fredricks,*† Jennifer A. Jolley,* Paul W. Lepp,* Jon C. Kosek,† and David A. Relman*† *Stanford University, Stanford, California, USA; and †Veterans Affairs, Palo Alto Health Care System, Palo Alto, California, USA Rhinosporidium seeberi, a microorganism that can infect the mucosal surfaces of humans and animals, has been classified as a fungus on the basis of morphologic and histochemical characteristics. Using consensus polymerase chain reaction (PCR), we amplified a portion of the R. seeberi 18S rRNA gene directly from infected tissue. Analysis of the aligned sequence and inference of phylogenetic relationships showed that R. seeberi is a protist from a novel clade of parasites that infect fish and amphibians. Fluorescence in situ hybridization and R. seeberi-specific PCR showed that this unique 18S rRNA sequence is also present in other tissues infected with R. seeberi. Our data support the R. seeberi phylogeny recently suggested by another group. R. seeberi is not a classic fungus, but rather the first known human pathogen from the DRIPs clade, a novel clade of aquatic protistan parasites (Ichthyosporea). Rhinosporidiosis manifests as slow-growing, that has been difficult to classify. Recently, tumorlike masses, usually of the nasal mucosa or R. seeberi has been considered a fungus, but it was ocular conjunctivae of humans and animals. originally thought to be a protozoan parasite (2). Patients with nasal involvement often have Its morphologic characteristics resemble those of unilateral nasal obstruction or bleeding due to Coccidioides immitis: both organisms have polyp formation. The diagnosis is established by mature stages that consist of large, thick-walled, observing the characteristic appearance of the organism in tissue biopsies (Figure 1). -

Fungal Infections (Mycoses): Dermatophytoses (Tinea, Ringworm)
Editorial | Journal of Gandaki Medical College-Nepal Fungal Infections (Mycoses): Dermatophytoses (Tinea, Ringworm) Reddy KR Professor & Head Microbiology Department Gandaki Medical College & Teaching Hospital, Pokhara, Nepal Medical Mycology, a study of fungal epidemiology, ecology, pathogenesis, diagnosis, prevention and treatment in human beings, is a newly recognized discipline of biomedical sciences, advancing rapidly. Earlier, the fungi were believed to be mere contaminants, commensals or nonpathogenic agents but now these are commonly recognized as medically relevant organisms causing potentially fatal diseases. The discipline of medical mycology attained recognition as an independent medical speciality in the world sciences in 1910 when French dermatologist Journal of Raymond Jacques Adrien Sabouraud (1864 - 1936) published his seminal treatise Les Teignes. This monumental work was a comprehensive account of most of then GANDAKI known dermatophytes, which is still being referred by the mycologists. Thus he MEDICAL referred as the “Father of Medical Mycology”. COLLEGE- has laid down the foundation of the field of Medical Mycology. He has been aptly There are significant developments in treatment modalities of fungal infections NEPAL antifungal agent available. Nystatin was discovered in 1951 and subsequently and we have achieved new prospects. However, till 1950s there was no specific (J-GMC-N) amphotericin B was introduced in 1957 and was sanctioned for treatment of human beings. In the 1970s, the field was dominated by the azole derivatives. J-GMC-N | Volume 10 | Issue 01 developed to treat fungal infections. By the end of the 20th century, the fungi have Now this is the most active field of interest, where potential drugs are being January-June 2017 been reported to be developing drug resistance, especially among yeasts. -

Oral Fungal Infections Diagnosis and Management
Oral Fungal Infections Diagnosis and Management a,b,c,d,e, f,g b,c,h David R. Telles, DDS *, Niraj Karki, MD , Michael W. Marshall, DDS, FICD KEYWORDS Candidiasis Oral thrush Oral fungal infection Mucormycosis Histoplasmosis Blastomycosis Aspergillosis Geotrichosis KEY POINTS Most solitary or primary oral fungal infections are rare with the exception of oral candidiasis. Candidiasis is the leading infection that most dental practitioners will see in clinical practice. Unless diagnosed early and treated aggressively, mucormycosis can be a locally invasive and disfiguring oral and maxillofacial fungal infection. This review includes several oral and maxillofacial fungal infections, including mucormy- cosis, candidiasis, aspergillosis, blastomycosis, histoplasmosis, cryptococcosis, and coccidioidomycosis. INTRODUCTION Fungal infections are of great concern in dentistry. Patients may present with infections that can be superficial or indicative of a more serious systemic illness. This article focuses on fungal infections that can range from primary (superficial) to disseminated infections that have a high mortality. Included in the review are the most common oral and maxillofacial fungal infections, route of spread, diagnosis, treatment as well The authors have nothing to disclose. a Oral & Maxillofacial Surgery, Herman Ostrow USC School of Dentistry, 925 West 34th, Street, Los Angeles, CA 90089, USA; b Oral & Maxillofacial Surgery, Private Practice: Huntington Beach Oral & Maxillofacial Surgery, 7677 Center Avenue, Suite 206, -

Conjunctival Candidiasis Mimicking Ocular Surface Squamous Neoplasia
Conjunctival Candidiasis Mimicking Ocular Surface Squamous Neoplasia Zhiyu Peng Fudan University Eye Ear Nose and Throat Hospital https://orcid.org/0000-0001-8318-8878 Jiang Qian Fudan University Eye Ear Nose and Throat Hospital Yinan Han ( [email protected] ) Fudan Eye & ENT Hospital, Shanghai Brief report Keywords: conjunctivitis, candidiasis, mycosis, squamous cell neoplasm Posted Date: July 13th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-691327/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/8 Abstract Purpose To report a case of conjunctival candidiasis mimicking ocular surface squamous neoplasia. Case presentation A 71-year-old man presented with a history of persistent redness, swelling and watering in the left eye accompanying an enlarging mass in the conjunctiva. He underwent excisional biopsy which showed granulomatous inammation accompanied by irregular and atypical squamous epithelium hyperplasia. Periodic acid-Schiff stain and methenamine silver stain revealed a fungi infection. Further secretion smear was performed to clarify the pathogen as Candida albicans and a chronic fungal maxillary sinusitis was found through imaging test. Thus a diagnosis of conjunctival candidiasis was made. Conclusions Conjunctivitis caused by fungi is rare and a trigger such as agriculture trauma, immunocompromise state, contact history to fungal environment or contaminated water or infection of adjacent organs occurs in most cases. We report the case not only to share diagnostic and treatment experience, but also describe the unique histopathological manifestation leading to a speculation that chronic fungal or candida albicans infection might induce squamous metaplasia. Introduction Ocular fungal infection is a sight-threating disease with multiple incentives typically involving the cornea and internal structures of the eye. -
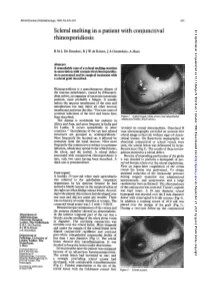
Scleral Melting in a Patient with Conjunctival Br J Ophthalmol: First Published As 10.1136/Bjo.74.10.635 on 1 October 1990
BritishJournal ofOphthalmology, 1990,74,635-637 635 Scleral melting in a patient with conjunctival Br J Ophthalmol: first published as 10.1136/bjo.74.10.635 on 1 October 1990. Downloaded from rhinosporidiosis R M L De Doncker, R J W de Keizer, J A Oosterhuis, A Maes Abstract A remarkable case ofa scleral melting reaction in association with conjunctival rhinosporidio- sis is presented and its surgical treatment with a scleral graft described. Rhinosporidiosis is a granulomatous disease of the mucous membranes, caused by Rhinospori- dium seeberi, an organism ofuncertain taxonomic position, most probably a fungus. It usually infects the mucous membranes of the nose and nasopharynx but may infect all other mucous membranes and even the skin.' Very rare cases of systemic infections of the liver and bones have been described. Figure I Scleral lesion (white arrow) and subepithelial The disease is worldwide but endemic in conjunctival bodies (black arrow). Africa and Asia, and most frequent in India and Sri Lanka. It occurs sporadically in other revealed no retinal abnormalities. Diascleral B countries."A Inoculations of the eye and related scan ultrasonography provided an acoustic-free structures are grouped as oculosporidiosis.2 scleral image at that site without signs of chorio- Most frequently the lacrimal sac is affected by retinal lesions. On fluorescein angiography no extension from the nasal mucosa. Next most abnormal conjunctival or scleral vessels were frequently the conjunctiva is subject to a primary seen; the scleral lesion was delineated by hypo- infection, which may spread to the orbital tissue, fluorescence (Fig 3). The results of these investi- the sclera, and the eyelids. -

The Taxonomic Status of Lacazia Loboi and Rhinosporidium Seeberi Has Been Finally Resolved with the Use of Molecular Tools Leonel Mendoza1, Libero Ajello2 & John W
Forum micológico Rev Iberoam Micol 2001; 18: 95-98 95 The taxonomic status of Lacazia loboi and Rhinosporidium seeberi has been finally resolved with the use of molecular tools Leonel Mendoza1, Libero Ajello2 & John W. Taylor3 1Medical Technology Program, Department of Microbiology, Michigan State University, East Lansing MI, 2School of Medicine, Emory University, Atlanta GA, and 3Department of Plant and Microbial Biology, University of California, Berkeley, CA, USA The etiologic agents of lobomycosis and rhinospo- fungal pathogen Paracoccidioides brasiliensis. More than ridiosis, Lacazia loboi and Rhinosporidium seeberi res- 70 years later, however, his hypothesis was still awaiting pectively, have long been confined in a taxonomic limbo. verification. Although their invasive cells in the tissues of infected To resolve this taxonomic standoff, a team of humans and other animals are abundant and readily detec- scientists from Sri Lanka, where R. seeberi infections are table histologically, they have been intractable to isolation common, and Brazil, where lobomycosis is endemic, and and culture. Thus, it has not been possible to objectively the USA was assembled to study R. seeberi’s and place them in any of the kingdoms in which all living enti- L. loboi’s phylogenetic links with other eukaryotic orga- ties are classified. In addition to their uncultivatable sta- nisms. The strategy was to collect fresh tissue samples tus, L. loboi and R. seeberi shared in common from cases of lobomycosis and rhinosporidiosis, isolate morphological features that resemble those of certain pat- genomic DNAs and then amplify their 18S small subunit hogenic fungi and protozoans: viz. essentially similar (SSU) rDNA by using standard primers and PCR techno- inflammatory host reactions, unresponsiveness to antifun- logy. -

Fungal Infections
Fungal infections Natural defence against fungi y Fatty acid content of the skin y pH of the skin, mucosal surfaces and body fluids y Epidermal turnover y Normal flora Predisposing factors y Tropical climate y Manual labour population y Low socioeconomic status y Profuse sweating y Friction with clothes, synthetic innerwear y Malnourishment y Immunosuppressed patients HIV, Congenital Immunodeficiencies, patients on corticosteroids, immunosuppressive drugs, Diabetes Fungal infections: Classification y Superficial cutaneous: y Surface infections eg. P.versicolor, Dermatophytosis, Candidiasis, T.nigra, Piedra y Subcutaneous: Mycetoma, Chromoblastomycosis, Sporotrichosis y Systemic: (opportunistic infection) Histoplasmosis, Candidiasis Of these categories, Dermatophytosis, P.versicolor, Candidiasis are common in daily practice Pityriasis versicolor y Etiologic agent: Malassezia furfur Clinical features: y Common among youth y Genetic predisposition, familial occurrence y Multiple, discrete, discoloured, macules. y Fawn, brown, grey or hypopigmented y Pinhead sized to large sheets of discolouration y Seborrheic areas, upper half of body: trunk, arms, neck, abdomen. y Scratch sign positive PITYRIASIS VERSICOLOR P.versicolor : Investigations y Wood’s Lamp examination: y Yellow fluorescence y KOH preparation: Spaghetti and meatball appearance Coarse mycelium, fragmented to short filaments 2-5 micron wide and up to 2-5 micron long, together with spherical, thick-walled yeasts 2-8 micron in diameter, arranged in grape like fashion. P.versicolor: Differential diagnosis y Vitiligo y Pityriasis rosea y Secondary syphilis y Seborrhoeic dermatitis y Erythrasma y Melasma Treatment P. versicolor Topical: y Ketoconazole , Clotrimazole, Miconazole, Bifonazole, Oxiconazole, Butenafine,Terbinafine, Selenium sulfide, Sodium thiosulphate Oral: y Fluconazole 400mg single dose y Ketoconazole 200mg OD x 14days yGriseofulvin is NOT effective. -

Candidemia in Critically Ill Patients: Comparison in IC VS
Histopathological diagnosis Arunaloke Chakrabarti 2017 Professor & Head Center for Advanced Research in Medical Mycology & WHO Collaborating Center Histopathological diagnosis Department of Medical Microbiology Postgraduate Institute of Medical EducationAUG & Research Professor Arunaloke Chakrabarti Chandigarh – 160012, India Head, Department of Medical Microbiology Postgraduate Institute of Medical Education and Research Chandigarh, India 5-6 Co-chair, ISHAM Asia Fungal Working Group SPEAKER CONFERENCE,OF MMTN Need of histopathological diagnosis Learning histopathological diagnosis • Fungi are ubiquitous & saprophyticAT • Learn tissue reaction to fungi • Usual laboratory contaminant COPYRIGHT • Commonly performed H & E stain in histopathology will help you to • Infection versus colonization - a frequent problem observe tissue reaction • Rapid & cost-effective means of providing a presumptive or • Difficult to identify fungi on H & E (except Histoplasma, Mucor etc.) definitive diagnosis of an invasive fungal infection • On H&E, all fungi show pink cytoplasm, blue nuclei & no colouration of • Help in knowing the load, tissue reaction, extent & invasion the wall. You may see unstained area in the position of fungi • Difficulty – to get samples from deep tissue • Go ahead performing PAS, GMS • Advances in diagnostic radiology & patient support (platelet • Other specific stains – Alcian blue, Mucicarmine, Fontana Masson etc. transfusions) have improved collection of tissue biopsy specimens PRESENTED • Need training…. 1 Other challenges • -

Yesterday, Today and Tomorrow of Mycology Editorial Imedpub Journals
Editorial iMedPub Journals Medical Mycology: Open Access 2016 http://www.imedpub.com/ Vol.2 No.3:e1 ISSN 2471-8521 DOI: 10.21767/2471-8521.1000e1 Yesterday, Today and Tomorrow of Mycology Habip Gedik* Department of Infectious Diseases and Clinical Microbiology, Ministry of Health Bakırkoy Training and Research Hospital, Istanbul, Turkey *Corresponding author: Habip Gedik, Department of Infectious Diseases and Clinical Microbiology, Ministry of Health Bakırkoy Training and Research Hospital, Istanbul, Turkey, Tel: 00 90 505 336 27 70; E-mail: [email protected] Received date: August 04, 2016; Accepted date: August 16, 2016; Published date: August 26, 2016 Citation: Habip G (2016) Yesterday, Today and Tomorrow of Mycology. Med Mycol Open Access 2: 3. doi: 10.21767/2471-8521.1000e1. Copyright: © 2016 Habip G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Editorial after regional cases were reported and geographically emerged. Fungi are a part of environmental life. The use of yeasts Candidiasis (Candida, Debaryomyces, Kluyveromyces, dates back to Sumeria 7000 B.C for beer. Fungi essentially Meyerozyma, Pichia, etc.), Cryptococcosis (Cryptococcus produce beer, wine, citric acid, single cell protein, fodder yeast, neoformans), Aspergillosis (Aspergillus fumigatus, Aspergillus and baker’s yeast. Fungi had become objects of interest with a niger, etc.), Pseudallescheriasis (Scedosporium, Lomentospora), growing awareness as a material for botanical investigation at Zygomycosis (Mucormycosis, Rhizopus, Mucor, Rhizomucor, the beginning of 1900s. It had contributed to development of Lichtheimia, etc.), Hyalohyphomycosis (Penicillium, Biology, and Botany in the first fifty years of the 20th century.