AT Chemistry
POGIL Atomic Structure
Part I. Properties of Electromagnetic Radiation
Introduction
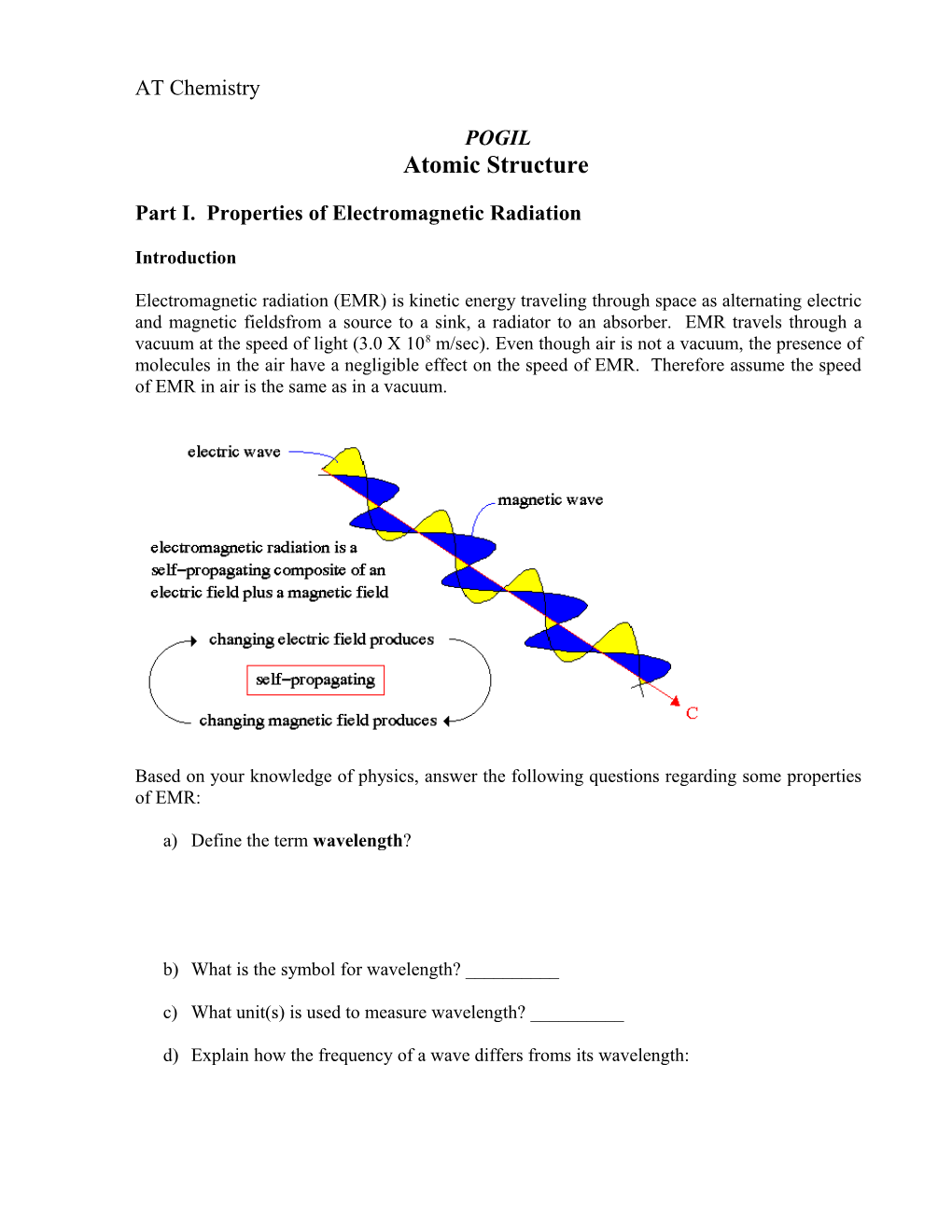
Electromagnetic radiation (EMR) is kinetic energy traveling through space as alternating electric and magnetic fieldsfrom a source to a sink, a radiator to an absorber. EMR travels through a vacuum at the speed of light (3.0 X 108 m/sec). Even though air is not a vacuum, the presence of molecules in the air have a negligible effect on the speed of EMR. Therefore assume the speed of EMR in air is the same as in a vacuum.
Based on your knowledge of physics, answer the following questions regarding some properties of EMR:
a) Define the term wavelength?
b) What is the symbol for wavelength? ______
c) What unit(s) is used to measure wavelength? ______
d) Explain how the frequency of a wave differs froms its wavelength: e) What symbol is used to denote requency? ______f) What is its unit of measure for frequency? ______g) What is the symbol for the speed of light? ______h) Describe the qualitative relationshiop between wavelength and frequency: i) Below is a diagram of the electromagnetic spectrum, which is divided into different regions based on differences in wavelengths and frequencies: note the wavelengthy is in meters.
Which region of the electromagnetic spectrum has the longest wavelengths and lowest frequencies? ______
What region of the electromagnetic spectrum has the shortest wavelengths and the highest frequencies? ______
In the visible region of the electromagnetic spectrum, which color of visible light has the highest frequency? ______
In the visible region of the electromagnetic spectrum, which color of visible light has the shortest wavelength? ______
Describe the approximate difference in magnitude between a microwave and and an X- ray.
2 j) Complete the following table:
Everyday Use of EMR Region of EMR Spectrum Listening to iPOD
Irradiation of food
Picture of your bones
suntanning
Night vision glasses (Predator vision)
k) To translate from the qualitative relationship to the quantitative relationship between two variables such as wavelength and frequency, a numeric constant is required. What constant is used to write a mathematical equation for the relationship of frequency and wavelength?
l) Write the mathematical equation that quantitatively relates wavelength and frequency:
Pause for class review
Part II. EMR Interaction with Matter
Reflection vs Absorption of EMR at Macroscopic Level (For Your Eyes Only)
Even though our Sun emits energy across the EMR spectrum, most the the energy given off is within the infrared, visible and ultraviolet regions of the EMR spectrum. Visible light can be thought of as white light, which consists of the ROYGBIV colors. Based on its chemical composition, an object will absorb and reflect specific wavelengths (colors) of visible light. The color of an object that an individual perceives is based on the wavelengths and therefore colors of light that are reflected by the object.
a) When exposed to white light, explain in terms of absorption and reflection of white light why;
A white object appears white:
3 A black object appears black:
A red object appears red:
b) What color will a red object appear in the presence of only blue wavelengths of light?
Problem 1
When sunlight shines on a green plant, the chlorophyll pigment in the leaves reflects wavelengths of green light and absorbs the other colors of visible light. One of the wavelengths is 500. nm. Calculate the frequency of this wavelength of light.
Problem 2
Your parents may listen to the “oldies but goodies” am station, WCBS FM, 101.1 MHz (megahertz). Calculate the wavelength in nanometers and to what region of the EMR spectrum does this wavelength correspond?
Pause for class review
Absoprtion vs Emission of EMR at the Atomic Level (enter the dynamic duo of Planck and Einstein)
Introduction
When a solid is heated until it “glows”, it emits electromagnetic radiation. The spectrum of light emitted by a heated solid is continuous (there are no missing frequencies) but it is not uniform. The intensity of radiation of radiation at some frequencies is much greater than the intensity at other frequencies. Classical physics (i.e. Newtonian physics) was unable to account for the spectra of heated solids known as “blackbody radiators”. Max Planck was able to account for the observation that light of some frequencies was emitted more intensely than light of other frequencies by postulating that the solid could emit energy only in certain discrete quantities. Planck proposed light energy travels in discrete packets of energy called quanta. This led to the
4 quantum theory of light. The energy of a quantum of light energy (called a photon) is directly proportional to its frequency, and the proportionality constant (h) is known as Planck’s constant. E = h
E = energy h = Planck’s constant = 6.63 X 10-34 Jsec = frequency
Based on the EMR Spectrum blow.
c) Which region of the electromagnetic spectrum corresponds to photons with the highest energy ______
d) Which region of the electromagnetic spectrum corresponds to photons with the lowest energy ______
e) Which color of visible EMR is composed of photons with the greatest amount of energy?
______
f) Explain how the wavelength and energy of a photon of EMR related?
g) Derive a mathematical equation that shows the relationship between the energy and the wavelength of a photon of EMR:
5 The term “radiation” has a negative connotation in everyday use as people believe that all types of radiation are dangerous. The radiation that is of concern to human health is ionizing radiation. This radiation has a high enough frequency and energy that can result in a biomolecule losing an electron and altering its chemical properties. The higher the frequency and energy of an EMR photon, the higher the ionizing ability and the higher the potential danger to human health. Not all EMR poses a threat to human health.
h) Based on energy of the photons, and your personal experiences, list two kinds of EMR that pose a potential human health threat.
Problem 3
i) X rays have wavelengths on the order of 1 X 10-10 m. Calculate the energy of 1.0 X 10-10 m X rays in units of kJ/mol of X rays.
j) AM radio waves have wavelengths on order of 1 X 104 m. Calculate the energy of 1.0 X 104 m radio waves in terms of Kj/mol of radiow waves.
k) Consider that the bond energy of a carbon-carbon single bond found in organic compounds is 347 kJ/mol. Would X rays and/or radio waves be able to disrupt organic compounds by disrupting organic compounds by breaking carbon-carbon sinle bonds?
Photoelectric Effect
6 Another observation which could not be explained through classical physics was the behavior of certain metals when exposed to light. Electrons are ejected from the surface of such a metal if the light has a frequency that is high enough. This is called the photoelectric effect. When the frequency is sufficiently high, the light provides enough energy to the electrons so they can overcome the forces that hold them within the metal. This amount of energy is called the “work function” of the metal. No matter how intense the light (i.e. if more photons/sec struck the metal), no matter how long the exposure, if the light had a frequency lower than the minimum value then it NEVER caused the ejection of electrons (just exactly what the minimum value was depended on the metal used). In contrast, even very low intensity beams of light that had a frequency higherr than the threshold did cause the effect and, moreover, it occurred immediately upon exposure. The electrons were able to escape provided that the radiation had a high enough frequency. The immediacy of the effect demonstrated that they obtained this energy almost simultaneously.
In 1905 Einstein provided the explanation: the metal was absorbing “particles” of light (photons), each having the energy
E = h
(i.e. it was frequency dependent and, like a particle, it arrived “all at once”). If the absorption of one photon provided sufficient energy, then the electron was ejected immediately. If a photon did not have enough energy, no ejection occurred because the energy could not be accumulated through multiple absorptions. If the photon possessed more energy than was required to free an electron, the electron carried off the excess as kinetic energy (it was ejected with a greater velocity). If the intensity was increased than the rate of ejection was increased simply because with more sufficiently energetic photons absorbed more electrons could be ejected in a given time interval.
Where do we see practical applications of the photoelectric effect? 7 Problem 4.
Light of a wavelength nof 4.00 X 102 nm strikes the surface of a sample of cesium metal. Electrons are ejected with a maximum kinetic energy of 1.54 X 10-19J.
l) Calculate the work function of cesium metal.
m) Determine the longest wavelength of light capable of ejecting electrons from cesium.
Part III. Atomic Spectra
White light is a combination of all frequencies in the visible region of the EMR spectrum (ROYGBIV). It is “continuous” in the sense that all frequencies of visible light are included in the spectrum. When gases or solids are heated (or “excited” some other way) until they glow, they re-emit the energy absorbed in the form of light of only certain frequencies, creating a spectrum that is not continous The explanation of these emission spectra, or bright line spectra, (i.e. why some frequencies but not others) from heated solids or gases could not be described through classical physics.
8 Recall that in the early 20th century the atomic model was the Rutherford atom, based on the famous gold-foil experiments. It was concluded that the atom consists of a dense, positively charged nucleus, with the electrons moving about outside the nucleus. The electrons were visualized as orbiting the nucleus, much as the planets orbit the sun. Unfortunately, this atomic model did not erxplain the frequencies of EMR emitted by electrons and the resulting emission spectra. In addition, it did not address the release of EMR that would occur when charged particles (electrons) are accelerating around the nucleus and would then cause energy to be released in the form of electromagnetic radiation (i.e. recall charged particles that accelerate must emit EMR photons). In fact, this is the principle that underlies our ability to transmit radio waves. Electrons are accelerated within an antenna, and electromagnetic radiation is emitted. An atom does not continually emit electromagnetic radiation. Moreover, if it did so, the electron would continue to lose energy and it would inevitaby spiral down into the nucleus. Thus, atoms would not be stable! But atoms are stable. Something was very wrong with Rutherford’s model. A new model – one consistent with observations – was needed.
Bohr Model
Niels Bohr provided a new model when he postulated that, yes, there were orbits, but only certain orbits were allowed, each having a distinct energy. Bohr was a student of Rutherford. Bohr’s model applied to the hydrogen atom with one electron and can be summarized as follows:
Electrons can only occupy certain specific circular orbits (i.e. principal energy levels.)
Electrons could pass from one allowed orbit to another by emitting or absorbing a photon that had an energy exactly equal to the difference between the two orbit energies. This means that orbits (principle energy levels are quantized (i.e. electrons each principle levels have specific energies). This is in keeping with the law of energy conservation.
Each photon that is emitted or absorbed has a specific amount of energy (or quantum), and therefore wavelength and frequency. Bohr observed that when the light emitted by a sample of hydrogen gas was passed through a prism and separated into its components, the spectrum that was produced contained only a few lines of different colors. He postulated that each colored line of light corresponded to a specific frequency of visible light which resulted from an electron transition between two different principal energy levels. Thus Bohr’s model explained the existence of bright line or emission spectra.
Define the following terms:
a) Ground State:
b) Excited State:
9 c) Continuous Spectrum:
d) Emission (bright line) spectrum:
e) Absorption (dark line) spectrum:
Bohr went on to show that for hydrogen (and ions having one electron), the electron could occupy only orbits in which the energy of the electron would fit the relation.
禳1 En = - RH 睚 铪n2
The negative sign is placed too indicate the energy of the electron is lower the closer it is to the nucleus compared to an infinite distance from the nucleus (where the energy = 0)
-18 Where n = 1,2,3, …. (priniple energy level), and RH = 2.18X 10 J (Rydberg constant)
If a transition occurs from a level having a principle quantum number ni to another level having a priniple quantum number nf, then the energy of the electron (and, hence, of the atom) will change by the amount
∆E = Ef - Ei
10 1 1 = -RH 睚 2- 2 nf n i
If the transition involves an absorption of energy, nf > ni and ∆E > 0
If the transition involves an emission of energy, nf < ni anf ∆E < 0
For the hydrogen atom:
When an electron drops from n = 7, 6, 5, 4, 3, 2 n = 1, the radiation emitted is ultraviolet and the spectral lines produced are referred to as the Lyman series.
When an electron drops from n = 7, 6, 5, 4, 3 n = 2, the radiation emitted is visible and the spectral lines produced are referred to as the Balmer series.
When an electron drops from n = 7, 6, 5, 4 n = 3, the radiation emitted is infrared and the spectral lines produced are referred to as the Paschen series.
In summary, 11 The Bohr model accounts for line spectra. Each line in a line spectrum is made by electromagnetic radiation of a particular frequency (ν). The radiation consists of photons of energy hν.
This energy is equal in magnitude to the enrergy difference (∆E) between two levels
(“orbits”) having principle quantum numbers ni and nf, between which a transition occurred.
Problem 1.
What is the wavelength of a photon emitted during a transition from the ni = 5 to the nf = 2 state in the hydrogen atom?
Problem 2.
Calculate the energy required to remove the electron from a hydrogen atom from its ground state.
Problems with Bohr Model:
- did not work for polyelectronic atoms
- Heisenberg showed electron could not really be located (we will discuss this later)
12 DeBroglie: demonstrated the wave-particle duality of matter.
f) Given the following two mathematical equations for energy relationships,
E = h ( = freqency) and E = mv2 (here we subsitute v, velocity, for c)
Derive a mathematical equation that allows you to calculate the wavelength of any object having a mass and velocity:
g) Based on the equation you derived above, explain why the wave-particle duality of matter is unimportant for macroscopic objects but very important when dealing with subatomic particles (like electrons):
Problem 3.
Calculate the wavelength of a 10 ton truck (1 ton = 2000 lbs, and 1 kg = 2.2 lbs) moving down kgm2 the state thruway at 65 mph (1km = 0.63 mile). . Note the unit of J = s2
13 Problem 4.
Calculate the wavelength of an electron traveling at 90% the speed of light. The mass of an electron = 9.11 X 10-31 kg
Heisenberg Uncertainty Principle:
Any method used to detect a subatomic particle will alter the position and momentum of that particle such that:
h X . (mv) > 4p
Where X = change in position
(mv) = change in momentum or energy
The more you know about the position of an electron, the less you know about its momentum (energy). This uncertainty does not apply to events on the macroscopic level. Heisenberg’s idea showed that Bohr’s idea of electrons at distinct positions around the nucleus could not be proven. In an orbit (principal energy level), both the position (distance from the nucleus) and momentum (product of mass and speed) would be known simultaneously (e.g. we always know these things for the Earth as it orbits the Sun). But that contradicts the uncertainty principle! So, electrons could not possibly occupy orbits (principal energy levels) as Bohr’s model claimed. The Bohr model had to be revised!
An alternate explanation to the Uncertainty Principle isthat any method used to detect an electron (like shinmg a photon on it) will in some way alter the position or energy of the electron is some unpredictable way.
Schrodinger - viewed electron as a "standing wave" around the nucleus. 14 In his model, only certain circular orbits have a circumference into which a whole number of wavelengths of the standing electron wave will "fit". He used complex mathematical functions to develop a “wave equation” that gave information for a wave function (). A specific wave function is called an orbital (e.g. 1s orbital). An orbital is a region in space around the nucleus with a 90% probability of finding a particular electron. In Schrodinger’s equation, the square of the function (2) indicates the probability of finding an electron at a particular point in space. Solving the wave equation yields four values, called quantum numbers – these describe properties of an orbital (or the most probable location of an electron).
Quantum Numbers n = the first (principal) number in the coordinate set and specify the energy level in which the electron is located. It is related to the size of the orbital. n the diagram on the following page the electron shown is in the second energy level, thus the principal quantum number is 2. In other words n tells us what energy level the electron is in. l = the second (or azimuthal) number is related to the angular momentum of the electron, which is determined by the shape of the orbital the electron is moving in. In the diagram the electron is moving in a spherical orbit, thus the l coordinate is assigned a value of 0. The l coordinate for a p (polar) orbital is 1, and the the d (diagonal) orbital the coordinate is 2, and for the f (fill in) orbital the value is 3. In other words, it tells us the sublevel (s, p, d or f) the electron is in. ml = magnetic position of the electron, which is related to the axis location of the electron. The electron in the diagram is in a spherical orbit and is thus assigned an axis value of 0. The assignments for the px, py, and pz values in the three polar orbitals is respectively, +1, 0, -1, and for the five diagonal axis of the d orbitals the values +2, +1, 0, -1, -2. In other words, this tells us which orbital in the sublevel the electron is located. ms = the electron spin of the electron around its own axis. The electron in the diagram is shown with its axis pointing up and will be assigned a spin value of +1/2. An electron may also be assigned a spin value of -1/2 which would be represented by an arrow pointing down. s orbitals 15 p orbitals
d orbitals
f orbitals 16 Schematic showing 1s, 2s, 2p, and 3s orboitals
The Periodic Table is arranged in such a way that the continual buildup of atoms and the quantum coordinates of every ground state electron is easily determined by its position in the table. Each row (period), column (group or family) and section of the periodic table (i.e. each element) can be assigned a uique set of four quantum numbers or coordinates.
Problem 5.
To check your understanding thus far, write the quantum numbers for the added valence electron labeled "A" and "B" in the blank periodic table below:
17 A
B
To review, the electron wave for each allowed energy state is called an orbital. Each orbital is described by three quantum numbers: n, l, and m . The fourth quantum number, m , l s distinguishes between each electron in an orbital.
QUANTUM NAME VALUES DESCRIPTION NUMBER ______
n Principal 1, 2, 3, ... orbital size and energy
l Azimuthal 0, 1, 2.....(n-1) orbital shape
s, l = 0 p, l = 1 d, l = 2 f, l = 3
ml Magnetic +l...0...-l orbital orientation
ms Spin +/- 1/2 spin of electron
18 Problem 5.
List the possible quantum numbers for a valence electron in phosphorous.
Problem 6.
What is the maximum number number of orbitals and electrons that can be described by the following set of quantum numbers:
n = 4, l = 3
Problem 7.
Explain whether the following sets of quantum numbers is possible: n = 3, l = 3, ml = 0, ms = -1/2
n = 2, l = 1, ml = -1, ms =-1
Problem 8. 19 Explain how a 2px differs from a 3px orbital:
Pauli Exclusion Principle
In a given atom no two electrons can have the same set of quantum numbers.
A Summary of the Hydrogen Atom
* In the wave mechanical model, the electron is viewed as a standing wave. This representation leads to a series of wave functions (orbitals) that describe the possible energies and spatial distributions available to the electron.
* In agreement with the Heisenberg uncertainty principle, the model cannot specify the detailed electron motions. Instead the square of the wave function represents the probability distribution of the electron in that orbital. This allows us to picture orbitals in terms of probability distributions, or electron density maps.
* The size of an orbital is arbitrarily defined as the surface that contains 90% of the total electron probability.
The hydrogen atom has many types of orbitals. In the ground state the single electron resides in the 1s orbital. The electron can be excited to higher-energy orbitals if energy is put into the atom. http://www.faculty.virginia.edu/consciousness/new_page_6.htm
20