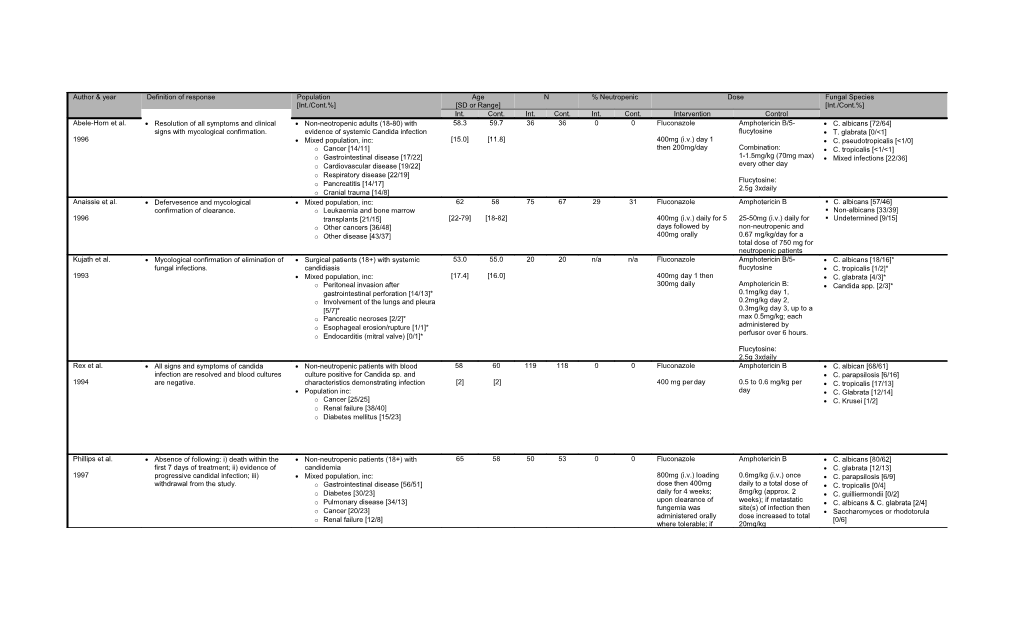
Author & year Definition of response Population Age N % Neutropenic Dose Fungal Species [Int./Cont.%] [SD or Range] [Int./Cont.%] Int. Cont. Int. Cont. Int. Cont. Intervention Control Abele-Horn et al. Resolution of all symptoms and clinical Non-neotropenic adults (18-80) with 58.3 59.7 36 36 0 0 Fluconazole Amphotericin B/5- C. albicans [72/64] signs with mycological confirmation. evidence of systemic Candida infection flucytosine T. glabrata [0/<1] 1996 Mixed population, inc: [15.0] [11.8] 400mg (i.v.) day 1 C. pseudotropicalis [<1/0] o Cancer [14/11] then 200mg/day Combination: C. tropicalis [<1/<1] o Gastrointestinal disease [17/22] 1-1.5mg/kg (70mg max) Mixed infections [22/36] o Cardiovascular disease [19/22] every other day o Respiratory disease [22/19] Flucytosine: o Pancreatitis [14/17] 2.5g 3xdaily o Cranial trauma [14/8] Anaissie et al. Defervesence and mycological Mixed population, inc: 62 58 75 67 29 31 Fluconazole Amphotericin B . C. albicans [57/46] confirmation of clearance. o Leukaemia and bone marrow . Non-albicans [33/39] 1996 transplants [21/15] [22-79] [18-82] 400mg (i.v.) daily for 5 25-50mg (i.v.) daily for . Undetermined [9/15] o Other cancers [36/48] days followed by non-neutropenic and o Other disease [43/37] 400mg orally 0.67 mg/kg/day for a total dose of 750 mg for neutropenic patients Kujath et al. Mycological confirmation of elimination of Surgical patients (18+) with systemic 53.0 55.0 20 20 n/a n/a Fluconazole Amphotericin B/5- C. albicans [18/16]* fungal infections. candidiasis flucytosine C. tropicalis [1/2]* 1993 Mixed population, inc: [17.4] [16.0] 400mg day 1 then C. glabrata [4/3]* o Peritoneal invasion after 300mg daily Amphotericin B: Candida spp. [2/3]* gastrointestinal perforation [14/13]* 0.1mg/kg day 1, o Involvement of the lungs and pleura 0.2mg/kg day 2, [5/7]* 0.3mg/kg day 3, up to a o Pancreatic necroses [2/2]* max 0.5mg/kg; each administered by o Esophageal erosion/rupture [1/1]* perfusor over 6 hours. o Endocarditis (mitral valve) [0/1]* Flucytosine: 2.5g 3xdaily Rex et al. All signs and symptoms of candida Non-neutropenic patients with blood 58 60 119 118 0 0 Fluconazole Amphotericin B C. albican [68/61] infection are resolved and blood cultures culture positive for Candida sp. and C. parapsilosis [6/16] 1994 are negative. characteristics demonstrating infection [2] [2] 400 mg per day 0.5 to 0.6 mg/kg per C. tropicalis [17/13] Population inc: day C. Glabrata [12/14] o Cancer [25/25] C. Krusei [1/2] o Renal failure [38/40] o Diabetes mellitus [15/23]
Phillips et al. Absence of following: i) death within the Non-neutropenic patients (18+) with 65 58 50 53 0 0 Fluconazole Amphotericin B C. albicans [80/62] first 7 days of treatment; ii) evidence of candidemia C. glabrata [12/13] 1997 progressive candidal infection; iii) Mixed population, inc: 800mg (i.v.) loading 0.6mg/kg (i.v.) once C. parapsilosis [6/9] withdrawal from the study. o Gastrointestinal disease [56/51] dose then 400mg daily to a total dose of C. tropicalis [0/4] o Diabetes [30/23] daily for 4 weeks; 8mg/kg (approx. 2 C. guilliermondii [0/2] o Pulmonary disease [34/13] upon clearance of weeks); if metastatic C. albicans & C. glabrata [2/4] fungemia was site(s) of infection then o Cancer [20/23] Saccharomyces or rhodotorula administered orally dose increased to total o Renal failure [12/8] [0/6] where tolerable; if 20mg/kg o Cardiovascular disease [4/6] metastatic infection, o Other [22/26] treatment extended to 8 weeks. van’t Wout et al. At least a 50% decrease in the size of the Neutropenic patients with proven or 51 32 16 16 100 100 Intraconazole Amphotericin B Candidiasis initial site of severity of the infection or suspected fungal infections Definite [19/6] 1991 complete clearance. Mixed population, inc: [15-74] [19-51] 200mg (oral) every 12 0.6mg/kg (i.v.) per day Probable [-/6] o Leukaemia [81/88] hours or 0.3mg/kg when in Possible [19/50] o Lymphoma [13/6] combination with o Others [6/6] flucytosine (150mg/kg) Aspergillosis Definite [6/13] Probable [44/19] Unknown [13/6]
Kullberg et al. Independent assessment of clinical and Patients (12+) with candida infection and 53.6 53.3 248 122 0 0 Voriconazole Amphotericin B C. albicans [43/51] mycological clearance at 12 weeks. who were non-neutropenic followed by fluconazole Non-albicans candida species 2005 [13-90] [13-87] 6mg/kg (i.v.) every 12 [61/50] hours for 24 hours 0.7-1.0mg/kg per day C. tropicalis [21/13] then 3mg/kg every 12 over 2-6 hours then C. parapsilosis [18/16] hours; after 3 days replaced by 400mg C. glabrata [15/17] patients could be fluconazole (i.v. or oral) C. krusei [2/1] switched to 200mg 2 x per day after a min 3 Other C. species [6/4] day (oral) days and max 7 days >2 candida species [5/3] Reboli et al. Global response (clinical and Patients (16+) with defined candidemia or 57.0 59.2 127 118 2 3 Anidulafungin Fluconazole C. albicans [64/59] microbiologic) at the end of other form of invasive candidiasis C. glabrata [16/25] 2007 intravenous therapy in patients who Mixed population, inc: [17.0] [16.5] 200mg on day 1 then 800mg (i.v.) day 1 then C. parapsilosis [10/14] 100 mg daily 400mg daily had a positive baseline culture. o Diabetes [35/25] C. tropicalis [12/9] o Renal failure/insuff [37/36] Other C. species [5/3] o Bacterial sepsis [46/42] o Neoplastic disease [22/23] o Disorders requiring transplant [6/4] Mora-Duarte et al. Resolution of all symptoms and signs of Patients (18+) with clinical evidence of 56 55 109 115 12.8 8.7 Caspofungin Amphotericin B C. albicans [35.6/54.1] candida infection and culture-confirmed infection and with positive cultures from C. parapsilosis [19.8/18.3] 2002 eradication (or presumptive eradication blood or other sterile site [17-84] [18-81] 70mg (i.v.) loading 0.6-0.7mg/kg (i.v.) per C. tropicalis [19.8/12.8] for certain nonblood infections). Mixed population, inc: dose then 50mg per day for patients without C. glabrata [12.8/9.2] o Diabetes mellitus [22.9/18.3] day neutropenia; 0.7-1.0 C. krusei [4.0/0.9] o Active leukaemia or lymphoma mg/kg for those with C. guilliermondii [3.0/0.9] neutropenia [14.7/11.3] C. lipolytica [1.0/0] o Renal failure or insufficiency C. rugosa [1.0/0] [21.1/26.1] Multiple spp. [3.0/3.7] o HIV infection [3.7/2.6] Kuse et al. Clinical and mycological confirmation of Patients (16+) with clinical signs of 54.5 56.0 247 247 13 10 Micafungin Liposomal amphotericin C. albicans [35/30] cleared invasive infection. systemic candida infections and positive B Non-albicans candida species 2007 cultures from blood or other sterile site [18-89] [16-97] 1-hour infusion of [49/43] Mixed population, inc: 100mg for patients 1-hour infusion of C. tropicalis [20/18] o Haematological disorder [19/13] >40kg of weight and 3mg/kg daily; after 5 C. parapsilosis [14/12] o Acute leukaemia [9/7] 2mg/kg for those days dose potentially C. glabrata [9/6] o Solid organ tumour [14/20] <40kg, daily; after 5 increased to 5mg/kg if C. krusei [2/2] days dose potentially mycological o Diabetes mellitus [12/12] C. guilliermondii [3/2] increased to 200mg if persistence. o Transplant [8/4] Other [2/5] mycological Non-fungal infection [7/5] o persistence. Multiple C. spp [6/4] o Gastrointestinal disorders [6/4] o HIV or other immune disorders [3/6] o Pancreatitis [3/4] o Renal failure [3/3] o Other [9/9] Pappas et al. Clinical and mycological success at the Predominantly nonneoplastic patients 56 (SD 55 (SD 390 188 6.9 3.7 Micafungin 100mg or Caspofungin 70mg on C. albicans {49/44} 2007 end of blinded intravenous therapy. Neutropenia [39/ 11] 16) 16) 150 mg once daily day one and 50mg per Non–C. albicans {52/60} Clinical success was defined as complete Recent surgery [113/76] intravenously day thereafter C. tropicalis {16/17} response to treatment (resolution of all Chemotherapy [64/20] C. glabrata {15/17} symptoms, signs, and abnormal radiographic findings). For patients with Hemodialysis [43/24] C. parapsilosis {12/22} candidemia, mycological success was Diabetes [132/48] C. krusei {4/4} defined as eradication if 2 cultures of Bacteremia [112/53] Other {6/5} blood specimens obtained at least 24h Pancreatitis [22/11] apart had negative results. Renal failure [116/58] Hepatic failure [10/6] Stem cell transplant [14/4] Organ transplant [16/7] Malignancy [124/52]
Legend: NA, Not available; int, intervention arm; cont, control arm; %, percentage; i.v., intravenous; *, absolute not percentage.