Ring-DCB Dienophile Synthon Strategy 1
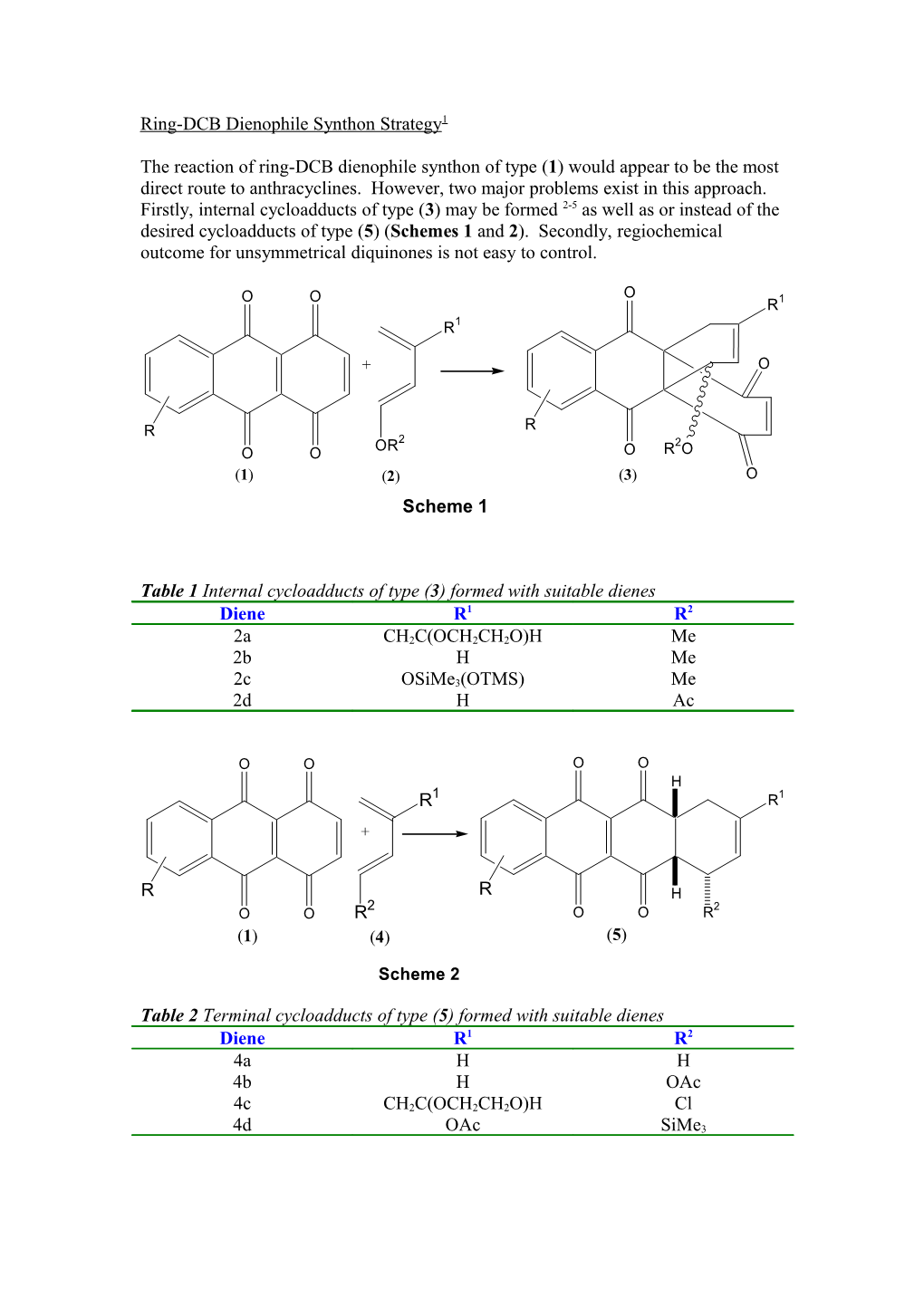
The reaction of ring-DCB dienophile synthon of type (1) would appear to be the most direct route to anthracyclines. However, two major problems exist in this approach. Firstly, internal cycloadducts of type (3) may be formed 2-5 as well as or instead of the desired cycloadducts of type (5) (Schemes 1 and 2). Secondly, regiochemical outcome for unsymmetrical diquinones is not easy to control.
O O O 1 R R1
+ O
R R OR2 2 O O O R O (1) (2) (3) O Scheme 1
Table 1 Internal cycloadducts of type (3) formed with suitable dienes Diene R1 R2
2a CH2C(OCH2CH2O)H Me 2b H Me 2c OSiMe3(OTMS) Me 2d H Ac
O O O O H R1 R1
+
R R H 2 2 O O R O O R (1) (4) (5)
Scheme 2
Table 2 Terminal cycloadducts of type (5) formed with suitable dienes Diene R1 R2 4a H H 4b H OAc 4c CH2C(OCH2CH2O)H Cl 4d OAc SiMe3 Generally, dienes that add to the internal double bond are electron-rich in character (2a – 2d). Conversely, dienes of type (4) that add2-5 to the external (terminal) double bond are either electron-deficient in character (4a –4d) or are unsubstituted (4a). The major cycloadducts are shown in Scheme 2. Note these are racemic mixtures.
Hückel perturbation molecular orbital calculations6 suggest that internal addition will be favoured by electron-rich dienes. This is due to the cumulative effect of the four carbonyl groups adjacent to the C-5a,9a double bond, thus making it more electron deficient and thereby decreasing it’s lowest unoccupied molecular orbital (LUMO) energy. The LUMO is more reactive towards electron-rich dienes of type (2a-d) which possess a high energy highest occupied molecular orbital (HOMO)7.
In my experimental work, oxidation of the bicyclic quinol [for compound (7) - see my website, http://www.jonathanpmiller.com] with lead(IV) acetate in acetic acid at room temperature afforded the tetraone (8) as green needles in 62% yield after crystallisation from chloroform-hexanes. Spectroscopic and microanalytical data were consistent for compound (8). The air-sensitive tetraone (8) was oxidized to the epoxytetraone (9) using m-chloroperoxybenzoic acid in dichloromethane at 0 oC to afford the compound (9) in 61% yield, after crystallisation from chloroform-hexanes.
O OH O O O O
O
O OH O O O O (7) (8) (9)
Reaction of the epoxytetraone (9) with the D-glucose-based diene (10) is shown in Scheme 3. The epoxytetraone (9) reacted with the diene (10) in acetone at 0-5 oC to afford mainly the cycloadduct (11) on the basis of limited 300 MHz 1H NMR spectroscopic and mass spectra data.
Unfortunately, attempted purification of compound (11) led to complications. The sample was first subjected to low-temperature silica-gel chromatography [with retention of regiostructure (11)]. Subsequent crystallisation from dichloromethane- diethyl ether-hexanes caused rearrangement of the cycloadduct (11) to it’s regioisomer (12), together with some hydolysis to the epoxypentaone (13) (islated in only 2% yield). The regioisomer (12) [11% based on the epoxytetraone (9) was the only identifiable component in the mother liquor. Spectroscopic data for these compounds were analogous to compounds listed in the website.
This synthesis resulted in compatively lower yields than using dienohile (7), but is analogous in steps to previously published work8-9 in the Dick Stoodley group. O O O O H t t OSiMe2Bu OSiMe2Bu O + O Acetone, 0 - 5 oC H O O O O O O OAc (9) OAc (11) O O
OAc OAc OAc OAc OAc OAc (10) Crystallisation 11% from (9) O O O O H H t O OSiMe2Bu
O O
H H O O O O O O
OAc OAc O O
OAc OAc
OAc OAc OAc OAc (13) (12) Scheme 3
References: 1. Adapted from the J. P. Miller, “Asymmetric Synthesis of Anticancer Anthracyclines”, Ph.D. Thesis, University of Manchester Institute of Science and Technology, pages 27-28, 1994. 2. T. R. Kelly, R. N. Goerner, J. W. Gillard and B. K. Prozak, Tetrahedron Letters, 1976, 3869. 3. T. R. Kelly and W. –G. Tsang, Tetrahedron Letters, 1978, 4457. 4. W. W. Lee, A. P. Martinez, T. H. Smith and D. W. Henry, J. Org. Chem., 1976, 41, 2296. 5. R. B. Garland, J. R. Palmer, J. A. Schultz, P. B. Sollman and R. Pappo, Tetrahedron Letters, 1978, 3669. 6. R. Sustmann, Tetrahedron Letters, 1971, 277. 7. I. Fleming, “Frontier Orbitals and Organic Chemical Reactions”, John Wiley and Sons, Cambridge, 1986, pages 86-181. 8. M. Chandler and R. J. Stoodley, J. Chem. Soc., Chem. Commun., 1978, 997. 9. R. C. Gupta P. A. Harland and R. J. Stoodley, Tetrahedron, 1984, 40, 4657.