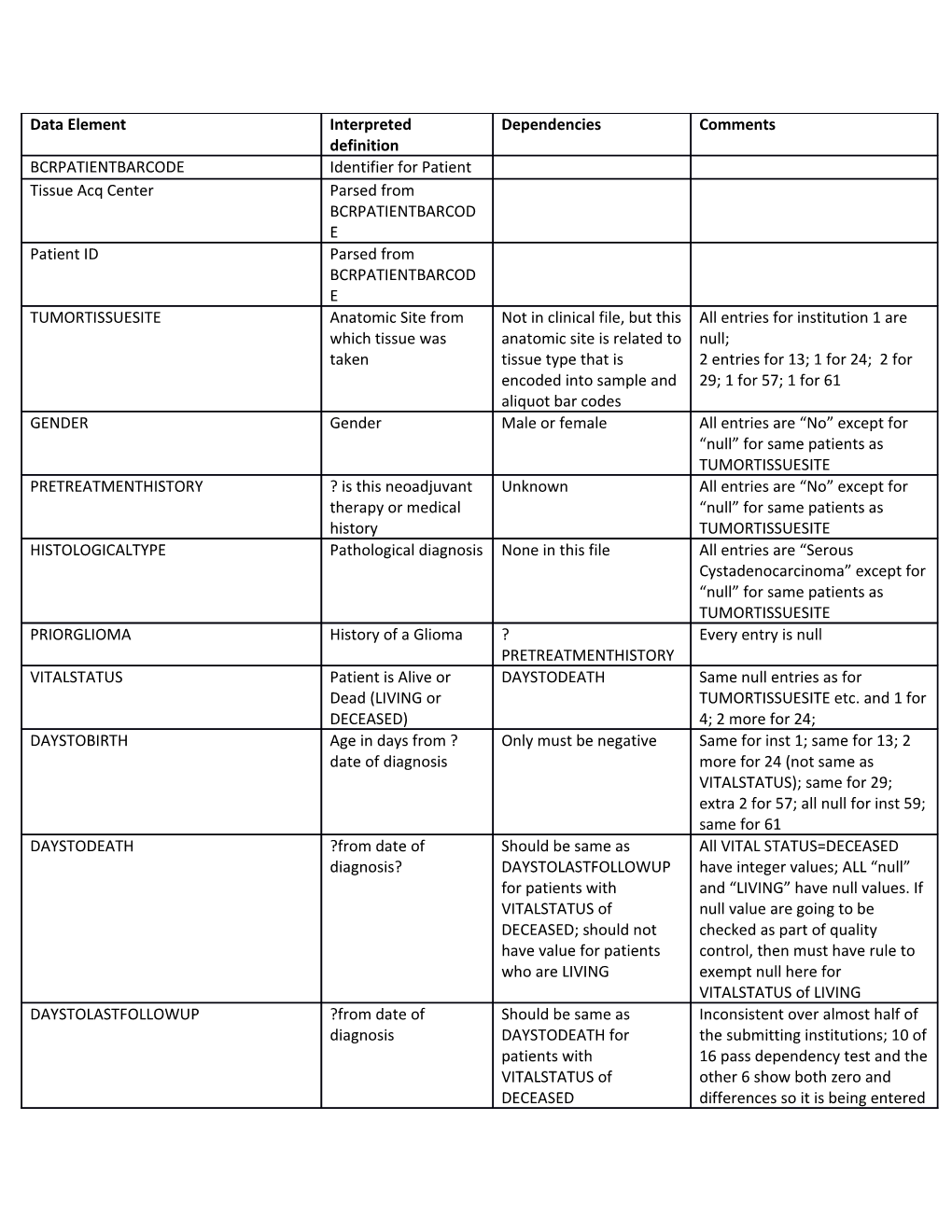
Data Element Interpreted Dependencies Comments definition BCRPATIENTBARCODE Identifier for Patient Tissue Acq Center Parsed from BCRPATIENTBARCOD E Patient ID Parsed from BCRPATIENTBARCOD E TUMORTISSUESITE Anatomic Site from Not in clinical file, but this All entries for institution 1 are which tissue was anatomic site is related to null; taken tissue type that is 2 entries for 13; 1 for 24; 2 for encoded into sample and 29; 1 for 57; 1 for 61 aliquot bar codes GENDER Gender Male or female All entries are “No” except for “null” for same patients as TUMORTISSUESITE PRETREATMENTHISTORY ? is this neoadjuvant Unknown All entries are “No” except for therapy or medical “null” for same patients as history TUMORTISSUESITE HISTOLOGICALTYPE Pathological diagnosis None in this file All entries are “Serous Cystadenocarcinoma” except for “null” for same patients as TUMORTISSUESITE PRIORGLIOMA History of a Glioma ? Every entry is null PRETREATMENTHISTORY VITALSTATUS Patient is Alive or DAYSTODEATH Same null entries as for Dead (LIVING or TUMORTISSUESITE etc. and 1 for DECEASED) 4; 2 more for 24; DAYSTOBIRTH Age in days from ? Only must be negative Same for inst 1; same for 13; 2 date of diagnosis more for 24 (not same as VITALSTATUS); same for 29; extra 2 for 57; all null for inst 59; same for 61 DAYSTODEATH ?from date of Should be same as All VITAL STATUS=DECEASED diagnosis? DAYSTOLASTFOLLOWUP have integer values; ALL “null” for patients with and “LIVING” have null values. If VITALSTATUS of null value are going to be DECEASED; should not checked as part of quality have value for patients control, then must have rule to who are LIVING exempt null here for VITALSTATUS of LIVING DAYSTOLASTFOLLOWUP ?from date of Should be same as Inconsistent over almost half of diagnosis DAYSTODEATH for the submitting institutions; 10 of patients with 16 pass dependency test and the VITALSTATUS of other 6 show both zero and DECEASED differences so it is being entered inconsistently within those institutions INFORMEDCONSENTVERIFIED Self-explanatory YES or should not be Same nulls as for visible TUMORTISSUETYPE except for inst 1 AGEATINITIALPATHOLOGICDIAGNOSIS Presumable years as Should correlate with 7 institutions are off by 1 year integer days to birth RADIATIONTHERAPY ?Primary adjuvant Should correlate with Same null distribution as therapy clinical_drug_public DAYSTO BIRTH CHEMOTHERAPY ?Primary adjuvant Should correlate with Same null distribution as therapy clinical_drug_public DAYSTO BIRTH. Apparently all patients received standard CHEMO as adjuvant therapy but some did receive other treatments following ADJUVANT IMMUNOTHERAPY ?Primary adjuvant Should correlate with Need better definition of therapy clinical_drug_public IMMUNOTHERAPY vs HORMONALTHERAPY vs TARGETEDMOLECULARTHERAPY, E.G. PT 1666 received oca rex oregovomab, an antibody targeted against ca-125, and this was entered as immunotherapy. This does not match NCI definition of immunotherapy HORMONALTHERAPY ?Primary adjuvant Should correlate with See above therapy clinical_drug_public TARGETEDMOLECULARTHERAPY ?Primary adjuvant Should correlate with See above therapy clinical_drug_public DAYSTOTUMORPROGRESSION ?is this tumor Patients with progression during DAYSTOPROGRESSION entries therapy do not have DAYSTORECURRENCE entries DAYSTOTUMORRECURRENCE ?is this defined as after therapy SITEOFTUMORFIRSTRECURRENCE Differentiated Should have entry for Some patients with entries for between METASTASIS DAYSTORECURRENCE DAYSTOPROGRESSION and not and LOCO-REGIONAL DAYSTO RECURRENCE have entries for SITEOFFIRSTTUMORRECURRENC E; this means that progression and recurrence do not have distinct definitions TUMORSTAGE AJCC Stage Null distribution almost exactly same as that for TISSUETUMORSITE TUMORGRADE AJCC grade Null distribution almost exactly same as that for TISSUETUMORSITE RESIDUALTUMOR This is rarely entered Has values of R1 and R0 R0 matches to No Macroscopic which apparently Disease value for correspond to No TUMORRESIDUALDISEASE; R1 Macroscopic Disease and matches to 1-10mm, 11-20mm NOT (No Macroscopic and >20 mm for Disease), respectively TUMORRESIDUALDISEASE all entries are null except for 1 for inst 57; 1 for inst 59; 2 for inst 13, 1 for isn’t 24, and 4 for isn’t 29 TUMORRESIDUALDISEASE Is this after surgery, Does not appear to cross- See slide after primary reference properly with adjuvant therapy, PRIMARYTHERAPY- after additional OUTCOMESSUCCESS therapy? PRIMARYTHERAPYOUTCOMESUCCESS Is this after surgery, or after primary adjuvant therapy ADDITIONALRADIATIONTHERAPY See discussion in ADDITIONALDRUGTHERAPY; lack of normalization or quality checks in the db ADDITIONALCHEMOTHERAPY ADDITIONALIMMUNOTHERAPY ADDITIONALHORMONETHERAPY ADDITIONALDRUGTHERAPY Should correlate with First glance shows problems therapy in where hormonal therapies such clinical_drug_public as tamoxifen and Fuluestrant are called Additional Hormonal Therapy in clinical_patient_public but are called targeted therapy in clinical_drug_public for patient with id 1350 at institution 4. Also note the type of therapy is typed in as Fuluestrant is Fulvestrant which is also called faslodex, as it is entered in other rows. Avastin is called immunotherapy in clinical_drug_public but it is a targeted therapy –this is just a quick review of patient 1350 from institution 4 ANATOMICORGANSUBDIVISION This would be Values are for laterality Since subsites do exist, it would anatomic subsite be preferable to separate subsite and laterality INITIALPATHOLOGICDIAGNOSISMETHO Is this supposed to be Values are mixed, e.g. FINE D the procedure upon NEEDLE ASPIRATION BIOPSY is which this was based based on CYTOLOGY or the type of assessment, but these are two pathology applied different values. Does cytology means washings performed during surgery? It would be best to define these with a pathologist. PERSONNEOPLASMCANCERSTATUS When-at date of last Should this have a Pt 1221 from Inst 4 is TUMOR followup? relationship to FREE after a CR but has a DAYSTORECURRENCE or METASTASIS after 1221 days; DAYSTOPROGRESSION Other patients from Inst 4 are WITH TUMOR after a CR but have non-zero or null DAYSTORECURRENCE and a SITEOFTUMORFIRSTRECURRENC E (so this field cannot be limited to after Primary Therapy); lack of normalization leads to multiple definitions and multiple contradictory entries.
Mary E. Edgerton MD, PhD Oct 7, 2010