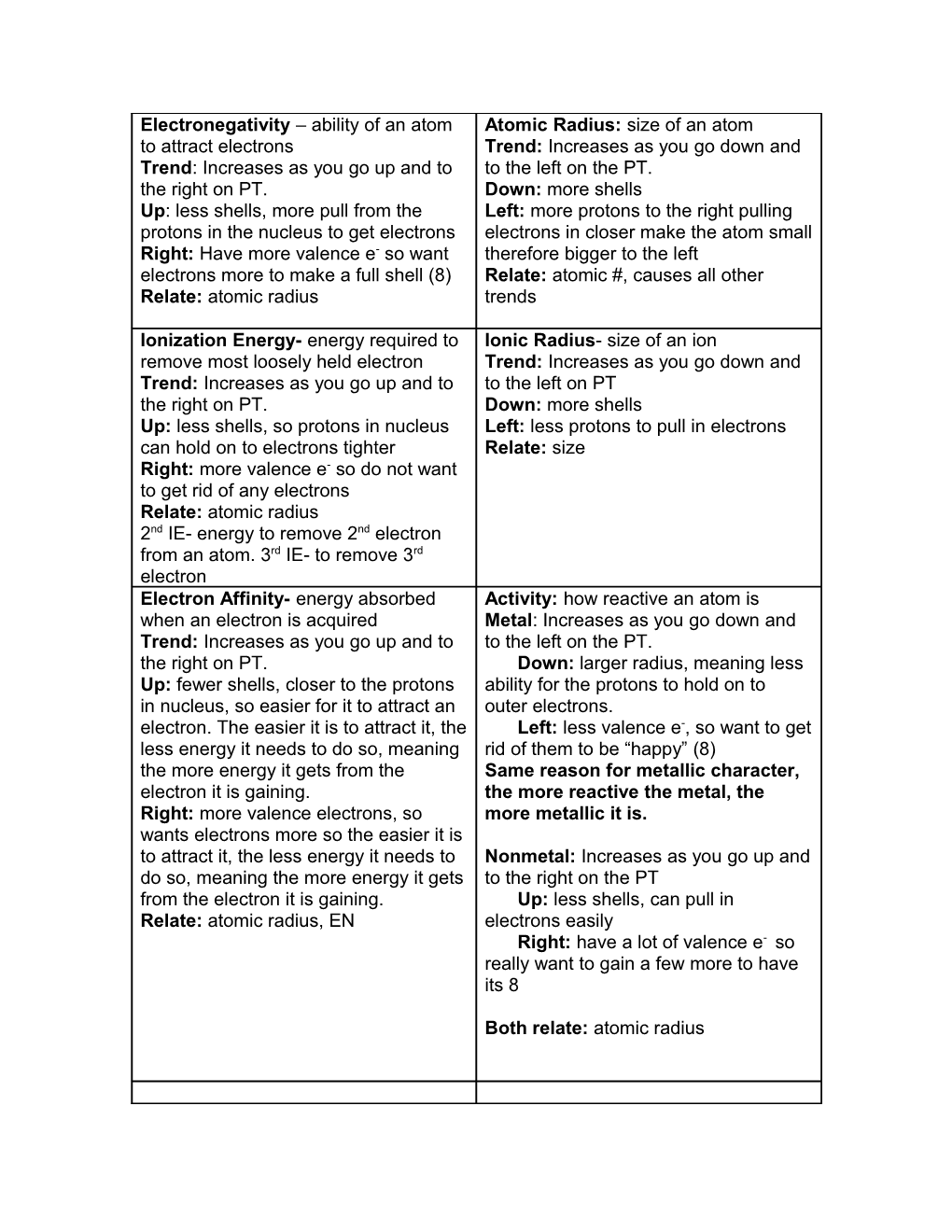
Electronegativity – ability of an atom Atomic Radius: size of an atom to attract electrons Trend: Increases as you go down and Trend: Increases as you go up and to to the left on the PT. the right on PT. Down: more shells Up: less shells, more pull from the Left: more protons to the right pulling protons in the nucleus to get electrons electrons in closer make the atom small Right: Have more valence e- so want therefore bigger to the left electrons more to make a full shell (8) Relate: atomic #, causes all other Relate: atomic radius trends
Ionization Energy- energy required to Ionic Radius- size of an ion remove most loosely held electron Trend: Increases as you go down and Trend: Increases as you go up and to to the left on PT the right on PT. Down: more shells Up: less shells, so protons in nucleus Left: less protons to pull in electrons can hold on to electrons tighter Relate: size Right: more valence e- so do not want to get rid of any electrons Relate: atomic radius 2nd IE- energy to remove 2nd electron from an atom. 3rd IE- to remove 3rd electron Electron Affinity- energy absorbed Activity: how reactive an atom is when an electron is acquired Metal: Increases as you go down and Trend: Increases as you go up and to to the left on the PT. the right on PT. Down: larger radius, meaning less Up: fewer shells, closer to the protons ability for the protons to hold on to in nucleus, so easier for it to attract an outer electrons. electron. The easier it is to attract it, the Left: less valence e-, so want to get less energy it needs to do so, meaning rid of them to be “happy” (8) the more energy it gets from the Same reason for metallic character, electron it is gaining. the more reactive the metal, the Right: more valence electrons, so more metallic it is. wants electrons more so the easier it is to attract it, the less energy it needs to Nonmetal: Increases as you go up and do so, meaning the more energy it gets to the right on the PT from the electron it is gaining. Up: less shells, can pull in Relate: atomic radius, EN electrons easily Right: have a lot of valence e- so really want to gain a few more to have its 8
Both relate: atomic radius