al_physical_formula (1/8)
Formula and Equation (1) Formula of Compounds The empirical formula of a compound is the formula, which shows the SIMPLEST whole number ratio of the number of atoms or ions present.
The molecular formula of a compound is the formula, which shows the ACTUAL number of each kind of atom in one molecule of compound.
The structure formula of a compound is the formula, which show how the constituents particles are joined up in the simplest unit of the compound.
For a molecular compound, the structural formula shows the arrangement of atoms in a molecule.
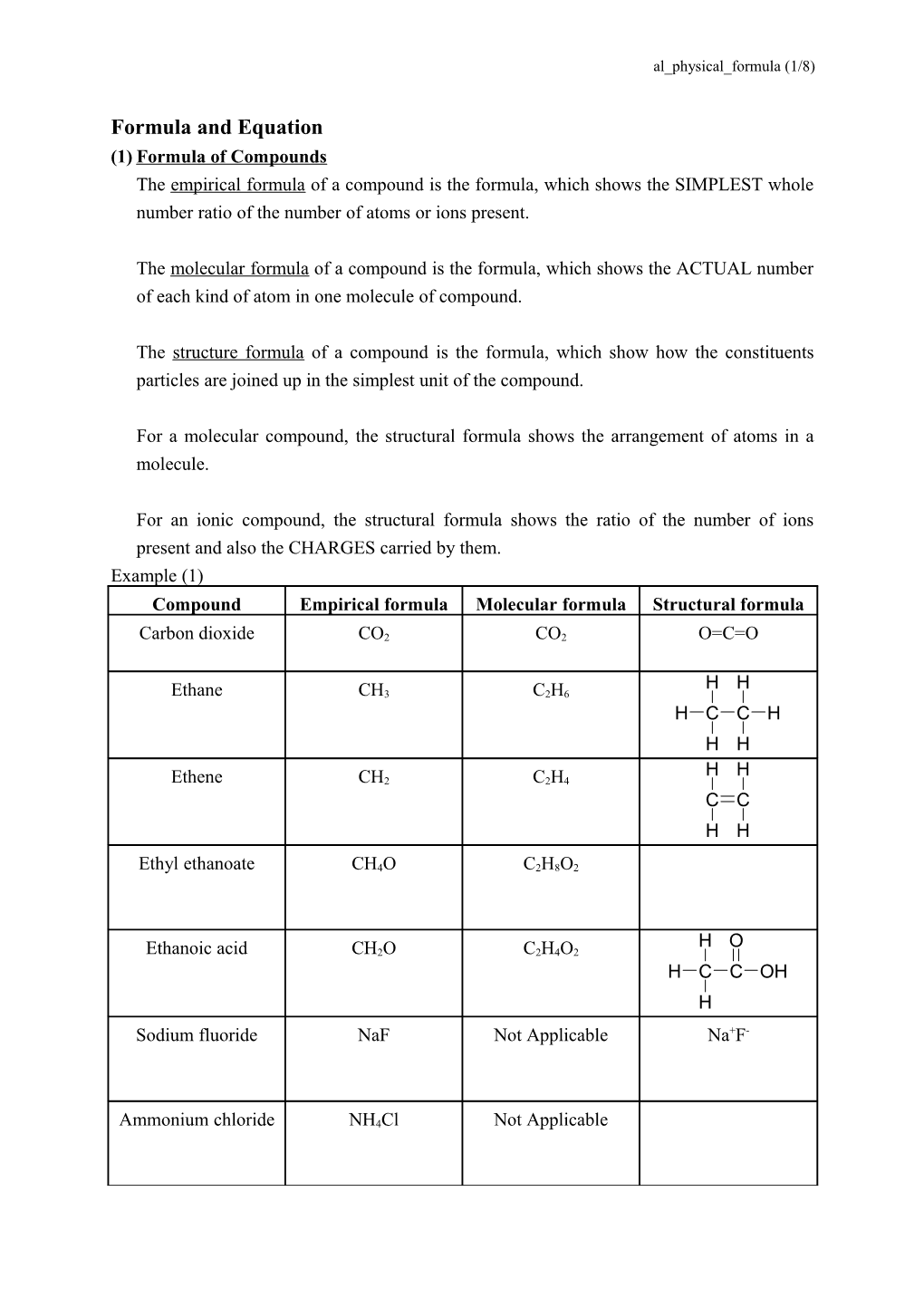
For an ionic compound, the structural formula shows the ratio of the number of ions present and also the CHARGES carried by them. Example (1) Compound Empirical formula Molecular formula Structural formula
Carbon dioxide CO2 CO2 O=C=O
H H Ethane CH3 C2H6 H C C H H H H H Ethene CH2 C2H4 C C H H
Ethyl ethanoate CH4O C2H8O2
H O Ethanoic acid CH2O C2H4O2 H C C OH H Sodium fluoride NaF Not Applicable Na+F-
Ammonium chloride NH4Cl Not Applicable al_physical_formula (2/8)
(2) Determination of formulae of simple compounds
(a) Finding the formula of black copper oxide
Excess town gas burning in small flame
Town gas
Black copper oxide Heat
Result: (i) Mass of the test tube = 18.100 g (ii) Mass of test tube + black copper oxide = 18.701 g (iii) Mass of test tube + copper = 18.579 g (iv) Mass of black copper oxide = g (v) Mass of oxygen in black oxide = g (vi) Mass of copper in black oxide = g
Calculations: (Relative atomic masses: Cu = 63.5, O = 16) Copper Oxygen Mass (g)
Number of mole
Mole ratio
Empirical formula of the copper oxide is ______al_physical_formula (3/8)
(b) Finding Formula from Composition by Mass
A compound X contains 80% carbon and 20% hydrogen by mass. Find its empirical formula. If the vapour density* of the gas is 15.0, find its molecular formula. (Relative atomic masses: C = 12, H = 1) Let the mass of X be 100g. Carbon Hydrogen Mass (g) 80 20
Number of mole 80/ 12 = 20/ 1 = 20
Mole ratio
Empirical formula of X: ______.
Molecular mass of X: 2 x 15 = 30
Molecular formula of X: ______.
*Vapour density of a gas = 1/2 x molecular mass of the gas density of the gas *Vapour density of a gas = density of hydrogen gas
Example (1) A compound containing only carbon, hydrogen and oxygen gave the following results on analysis: 0.81 g of substance gave 1.32 g of carbon dioxide and 0.45 g of water. Find the empirical formula of the compound. If its vapour density was 80, find also its molecular formula. al_physical_formula (4/8)
Example (2) A hydrated salt of magnesium contains 48.78% by mass of the anhydrous salt. The anhydrous salt has the following percentage composition by mass: magnesium = 20.00%; sulphur 26.67%; oxygen = 53.33%. Find the empirical formula of the hydrated salt.
(c) Finding composition by mass from formula
Example (1) Calculate the percentage by mass of copper, sulphur, oxygen and hydrogen in hydrated copper (II) sulphate, CuSO4 .5H2O. al_physical_formula (5/8)
(2) Chemical Equation Calculations based upon equations
(a) Reacting masses and volumes
Example (1) Calculate the mass of magnesium oxide formed when 2.43 g of magnesium was burnt with… (a) excess oxygen (b) 1.28 g of oxygen.
Given that: 2 Mg (s) + O2 (g) 2 MgO (s)
Example (2) Calculate the mass of copper produced by the complete reduction of 15.9 g of copper (II) oxide in hydrogen.
Given that: CuO (s) + H2 (g) Cu (s) + H2O (g) al_physical_formula (6/8)
Example (3) 5.91 g of iron was dissolved in excess diluted hydrochloric acid to give a solution containing Fe2+ ions. The solution was then boiled with concentrated nitric acid to oxidize all Fe2+ ions into Fe3+ ions. Excess sodium hydroxide solution was added to precipitate all Fe3+ ions as iron
(III) hydroxide, Fe(OH)3. The precipitate was filtered off, washed, dried and finally heated to convert all into iron (III) oxide, Fe2O3. Find mass of iron (III) oxide obtained. + 2+ 2+ 3+ - Given that: Fe + 2 H Fe + H2 Fe Fe + e 3+ - Fe + 3 OH Fe(OH)3 2 Fe(OH)3 Fe2O3 + 3 H2O
Example (4) Sodium hydrogen carbonate decomposes according to the equation:
2 NaHCO3 (s) Na2CO3 (s) + CO2 (g) + H2O (l) In order to obtain 36 cm3 of carbon dioxide (under room temperature and pressure), what is the minimum mass of sodium hydrogen carbonate used? (Molar volume of a gas at room temperature and pressure can be taken to be 24 dm3) al_physical_formula (7/8)
Example (5) 10.6 g of anhydrous sodium carbonate was added to 100 cm3 of 1.60 M HCl at 32 oC and 0.98 atm. Find the volume of gas evolved.
(b) Reacting volumes
Gay Lussac's Law: When gases react, they do so in volumes which bear a SIMPLE RATIO to one another, and to the volumes of the products if gaseous, all volumes being measured under the same conditions of temperature and pressure.
Example (1) Calculate the volume of oxygen required for the complete combustion of 100 cm3 of ethane,
C2H6. What is the volume of carbon dioxide and water formed, assuming all the volumes are measured at room temperature and pressure?
Given that: 2 C2H6 (g) + 7 O2 (g) 4 CO2 (g) + 6 H2O (l) al_physical_formula (8/8)
Example (2) 3 72.0 cm of a mixture of methane (CH4) and ethane (C2H4) was exploded with excess oxygen. The volume of the resulting gas was found to decrease by 96.0 cm3 when treated with concentrated sodium hydroxide solution (used to absorb carbon dioxide). Assuming constant pressure of 0.97 atm and temperature of 20.0 oC throughout, calculate the (a) composition by volume (b) number of moles of methane in the original mixture.
Example (3) 15 cm3 of a gaseous compound (containing only carbon and hydrogen) was mixed with 100 cm3 of oxygen which was in excess. The mixture was exploded and after cooling, the residual volume was 85 cm3. On adding concentrated aqueous potassium hydroxide, the volume decreased to 55 cm3. Calculate the molecular formula of the compound, assuming all volumes were measured under room conditions.