6 Worksheet 1
Amino acids
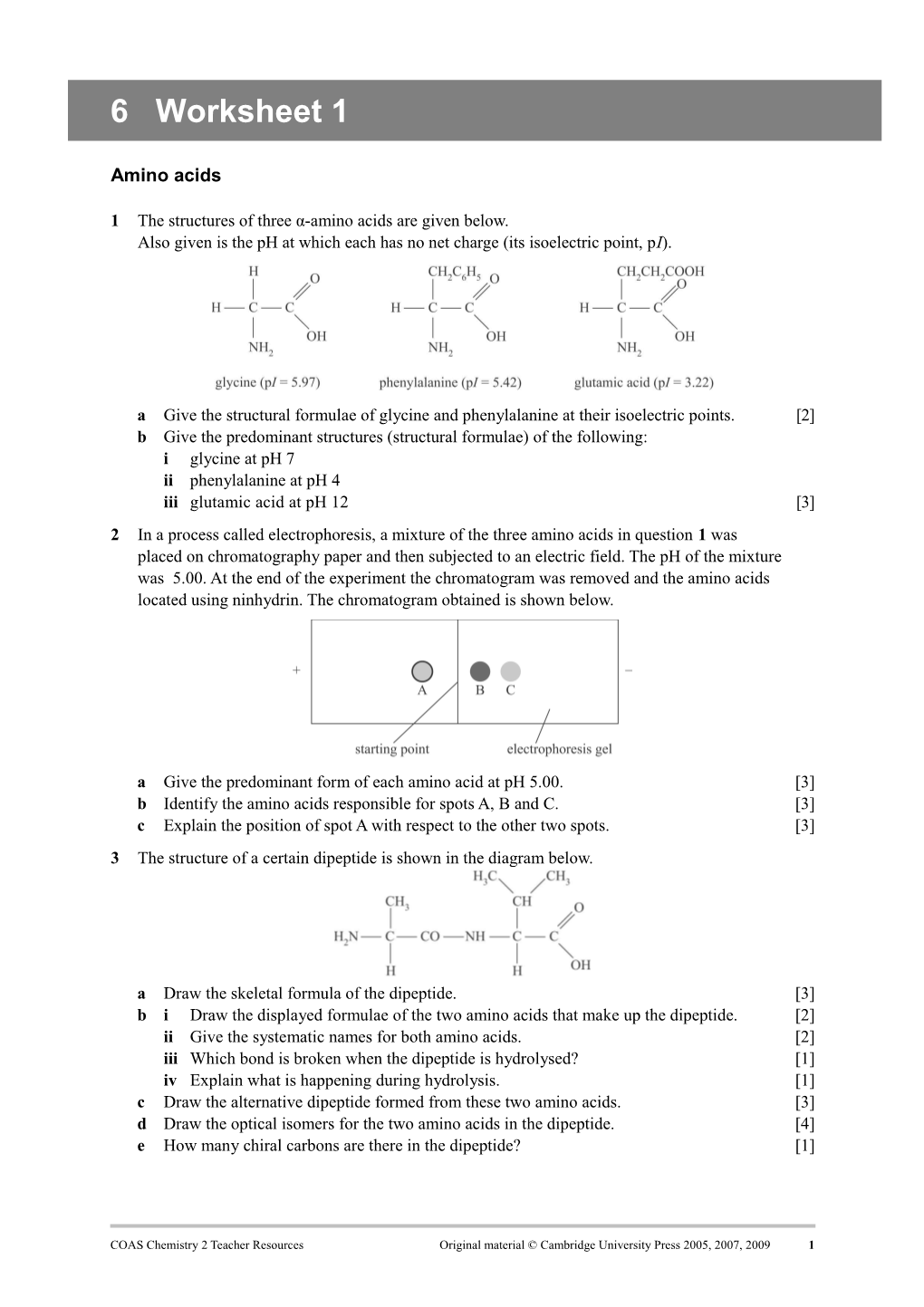
1 The structures of three α-amino acids are given below. Also given is the pH at which each has no net charge (its isoelectric point, pI).
a Give the structural formulae of glycine and phenylalanine at their isoelectric points. [2] b Give the predominant structures (structural formulae) of the following: i glycine at pH 7 ii phenylalanine at pH 4 iii glutamic acid at pH 12 [3] 2 In a process called electrophoresis, a mixture of the three amino acids in question 1 was placed on chromatography paper and then subjected to an electric field. The pH of the mixture was 5.00. At the end of the experiment the chromatogram was removed and the amino acids located using ninhydrin. The chromatogram obtained is shown below.
a Give the predominant form of each amino acid at pH 5.00. [3] b Identify the amino acids responsible for spots A, B and C. [3] c Explain the position of spot A with respect to the other two spots. [3] 3 The structure of a certain dipeptide is shown in the diagram below.
a Draw the skeletal formula of the dipeptide. [3] b i Draw the displayed formulae of the two amino acids that make up the dipeptide. [2] ii Give the systematic names for both amino acids. [2] iii Which bond is broken when the dipeptide is hydrolysed? [1] iv Explain what is happening during hydrolysis. [1] c Draw the alternative dipeptide formed from these two amino acids. [3] d Draw the optical isomers for the two amino acids in the dipeptide. [4] e How many chiral carbons are there in the dipeptide? [1]
COAS Chemistry 2 Teacher Resources Original material © Cambridge University Press 2005, 2007, 2009 1 4 Write the equations for the reactions between glycine (2-aminoethanoic acid, H2NCH2COOH) and the following reagents: a NaOH [2] b HCl [2] c another molecule of glycine [2]
5 The amino acid alanine is CH3CH(NH2)COOH. a Draw the displayed formula of alanine. [1] b Draw the displayed formula of alanine as a zwitterion. [1] c Draw the displayed formula of alanine as it will be at pH 2. [1] d Draw the displayed formula of alanine as it will be at pH 12. [1] e Draw a dipeptide made from two alanine molecules. Label the peptide linkage. [1] f How could you hydrolyse the dipeptide in part e? Include a balanced symbol equation in your answer. [3]
g The amino acid glycine is NH2CH2COOH. Two different dipeptides can be formed consisting of one alanine residue and one glycine residue. Draw the two dipeptides. [2] h Give two names for the natural condensation polymers formed by amino acids. [2]
Total: Score: % 49
COAS Chemistry 2 Teacher Resources Original material © Cambridge University Press 2005, 2007, 2009 2