KEY Practice Thermochemistry Test : 3.3-3.5, 10.5
Learning Goal: C-Level I can interpret the change in state and energy information contained in a heating/ cooling curve. C-Level I can explain the relationship between temperature and kinetic energy.
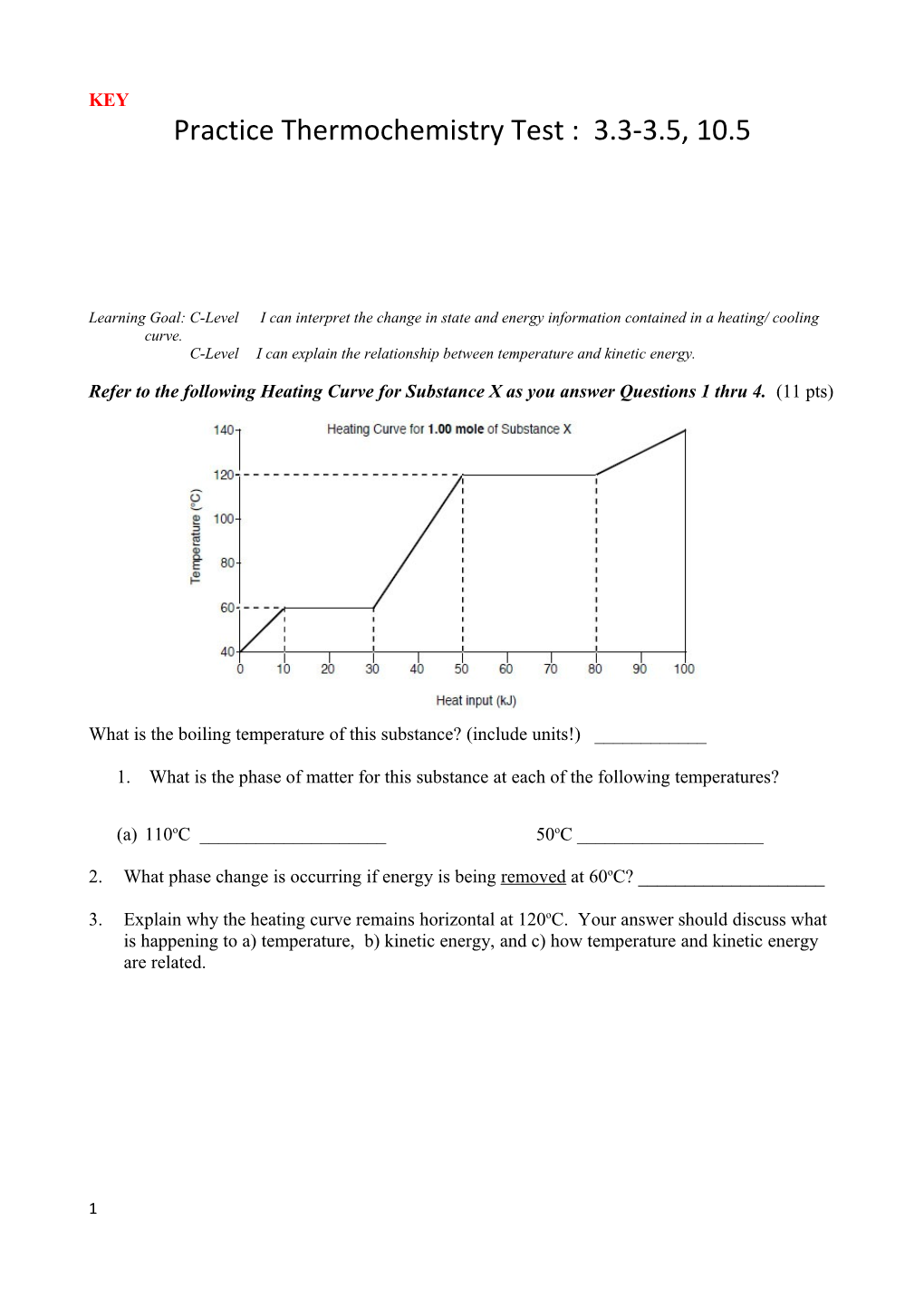
Refer to the following Heating Curve for Substance X as you answer Questions 1 thru 4. (11 pts)
What is the boiling temperature of this substance? (include units!) ______
1. What is the phase of matter for this substance at each of the following temperatures?
(a) 110oC ______50oC ______
2. What phase change is occurring if energy is being removed at 60oC? ______
3. Explain why the heating curve remains horizontal at 120oC. Your answer should discuss what is happening to a) temperature, b) kinetic energy, and c) how temperature and kinetic energy are related.
1 Learning Goal: B-Level I can calculate the heat of fusion or vaporization for a substance. B-Level I can use the heat equation to calculate the thermal energy changes of a substance.
4. Calculate the energy required, in kilojoules, for each of the following: Follow ALL math work rules!! (6 pts)
(a) The energy change when 20.0 g of steam is cooled from 120. oC to 100oC.
q = msteam SHsteam ΔTsteam q = 20.0 g 1.841 (100oC – 120oC)
q = 36.82 ─ 20 oC → q = ─ 736.4 J 1,000 = ─ 0.7364 kJ
(i) During this process is energy being absorbed or released? ______
(b) The energy change when 20.0 g of ice is melted.
= mice
= 20.0 g 334 = 6,680 J 1,000 = + 6.68 kJ
(i) During this process is energy being absorbed or released? ______
Learning Goal: C-Level I can explain the relationship between Ek and Heat (thermal) energy transfers between substances. C-Level I can explain internal energy and heat (thermal) energy of a substance.
5. (a) What is heat (thermal energy) and how is it different from temperature? (2 pts) Heat = energy which is being transferred from one object to another due to a temperature difference
Temperature = a measure of the average EK of an object
(b) What is internal energy? (2 pts) the sum of the EK and EP of all of the atoms of a substance
2 (c) How are heat and internal energy related? (2 pts) The more internal energy an object has the more “heat” it will have available to transfer Learning Goal: B-Level I can use the heat equation to calculate thermal energy changes of a substance. C-Level I can define the concept and compare the heat capacity and specific heat of various substances.
6. In the old days, on a cold winter night it was common to bring a hot object to bed with you. Which of the following objects – a 10-kilogram iron brick or a 2-kilogram jug of hot water – would be better to warm up your bed to a nice, cozy temperature of 25.0oC ? Use calculations as part of your answer. Write a few sentences to justify your choice. (7 points)
Don't forget to: (a) Use the concept of specific heat to clearly indicate WHICH SUBSTANCE would be the best choice (b) use CALCULATIONS to illustrate WHY this substance would be the best choice (c) write a few sentences in which you COMPARE the results of your calculations and HOW they justify your choice – your answer should include a comparison of the masses and energy of each substance and how this relates to keeping you warm
Specific Heat Mass ΔT Substance (kg) (oC) Water 2 4.184 25.0 Iron 10 0.45 25.0
(a) 2 kg of water would be the would be the best choice b/ c water has a higher specific heat than iron
(b) calculations to determine the energy which could be released by each substance
= ΔT
3 o i) q = 2000 g 4.184 25.0 C → 209,200 J of energy for water
o ii) q = 10,000 g 0.45 25.0 C → 112,500 J of energy for iron
(c) 2 kg of water can store more energy than 10 kg of iron (see work above.) The more energy it stores the longer it will be able to keep you warm.
Learning Goal: A-Level I can use the heat equation to calculate energy changes between two substances.
BONUS: A 35.2-g sample of a metal heated to 100.0oC is placed in a calorimeter containing 42.5 g of water at an initial temperature of 19.2oC. If the final temperature of the metal and the water is 29.5oC, what is the specific heat of the solid? st 1 : calculate ΔTwater o o o ΔT = Tf ─ Ti → 29.5 C − 19.2 C = 10.3 C
2nd : calculate energy gained by water o qwater = (42.5 g) (4.184 ) (10.3 C ) → 1812.29 J
rd 3 : remember that +qwater = ─qmetal
─qmetal = ─ 1812.29 J
th 4 : calculate cmetal o o ─ 1812.29 J = 35.2 g SHmetal (29.5 C – 100 C) = 0.730
Equation Sheet for Thermodynamics Test
4 5