BLOCK ______NAME ______
DETERMINING THE CALORIC CONTENT OF VARIOUS NUTS - LAB
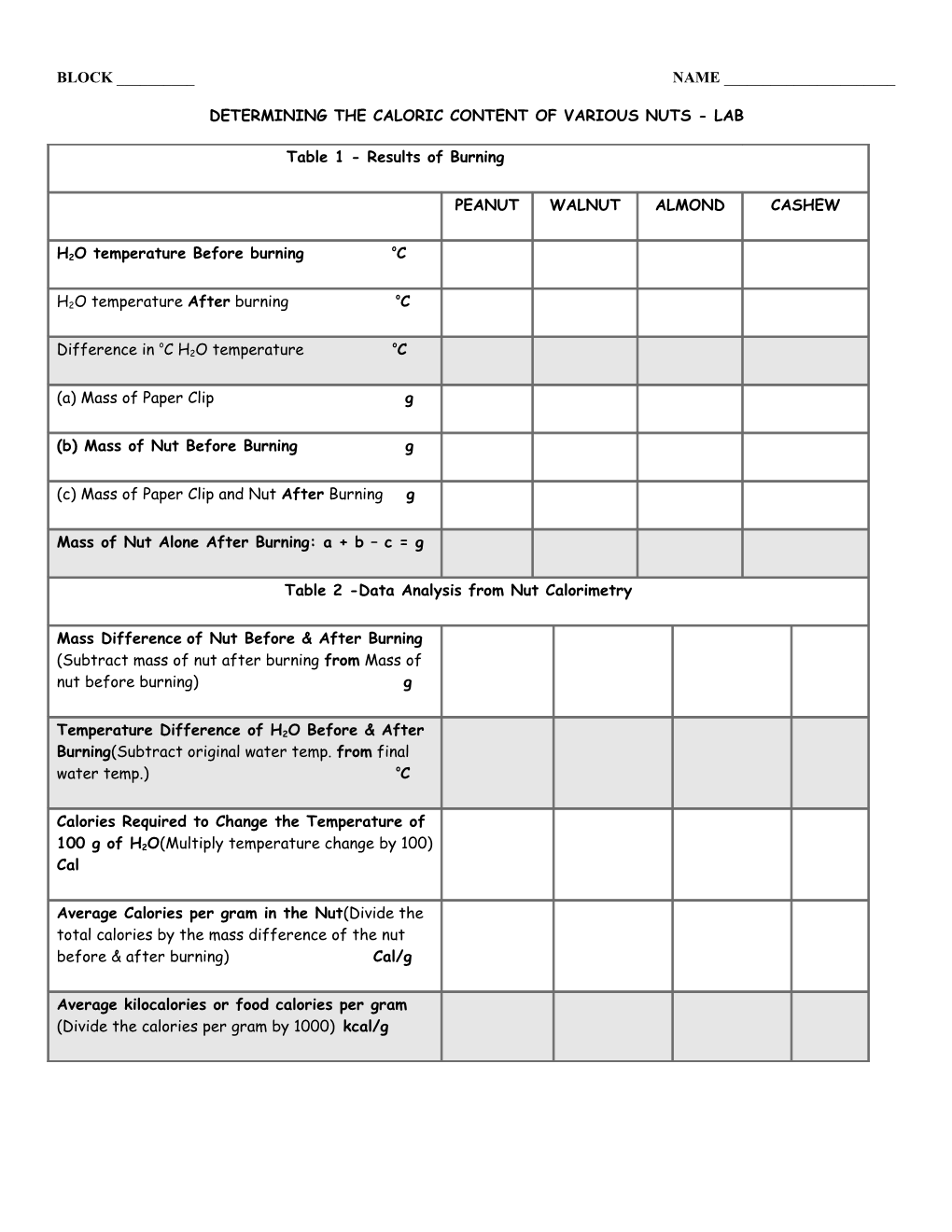
Table 1 - Results of Burning
PEANUT WALNUT ALMOND CASHEW
o H2O temperature Before burning C
o H2O temperature After burning C
o o Difference in C H2O temperature C
(a) Mass of Paper Clip g
(b) Mass of Nut Before Burning g
(c) Mass of Paper Clip and Nut After Burning g
Mass of Nut Alone After Burning: a + b – c = g
Table 2 -Data Analysis from Nut Calorimetry
Mass Difference of Nut Before & After Burning (Subtract mass of nut after burning from Mass of nut before burning) g
Temperature Difference of H2O Before & After Burning(Subtract original water temp. from final water temp.) oC
Calories Required to Change the Temperature of
100 g of H2O(Multiply temperature change by 100) Cal
Average Calories per gram in the Nut(Divide the total calories by the mass difference of the nut before & after burning) Cal/g
Average kilocalories or food calories per gram (Divide the calories per gram by 1000) kcal/g EXPERIMENTAL PROCEDURE
Obtain a metal can. If necessary, wipe any carbon deposits off the bottom of the can using a paper towel. Set up the apparatus as shown in figure below (please place a sheet of aluminum foil below work area).
Add 100 ml of water to the can. Determine the temperature of the water on degrees C. Remove thermometer from the can of water. Select a peanut (or other nut), weigh it, and record its mass. Shape a paper clip into a nut holder.
Record the mass of your paper clip nut holder. Place the peanut (or other nut used) on the stand and light it using a match or a burner. Move the can over the burning peanut and position it so that the top of the flame just touches the bottom of the can. Allow the peanut to burn completely. If the flame goes out and the peanut is not completely burned, relight the peanut. Remember to move the can away from over the peanut when relighting it. After the peanut has burned out, determine the new temperature of the water on degrees C. After the nut is burned, some charcoal remains. Carefully transfer the remaining material and paper clip holder to the scale and weigh them together. Determine the mass of the remaining material. The mass of peanut, or food, burned is equal to the initial mass of the peanut plus the mass of the paper clip, minus the mass of the remaining material and the paper clip. Repeat the procedure with the other nuts. Complete the Data Analysis for the lab