Course Outcomes Worksheet 4:014 Principles of Chemistry II Norbert J. Pienta, 2/13/01
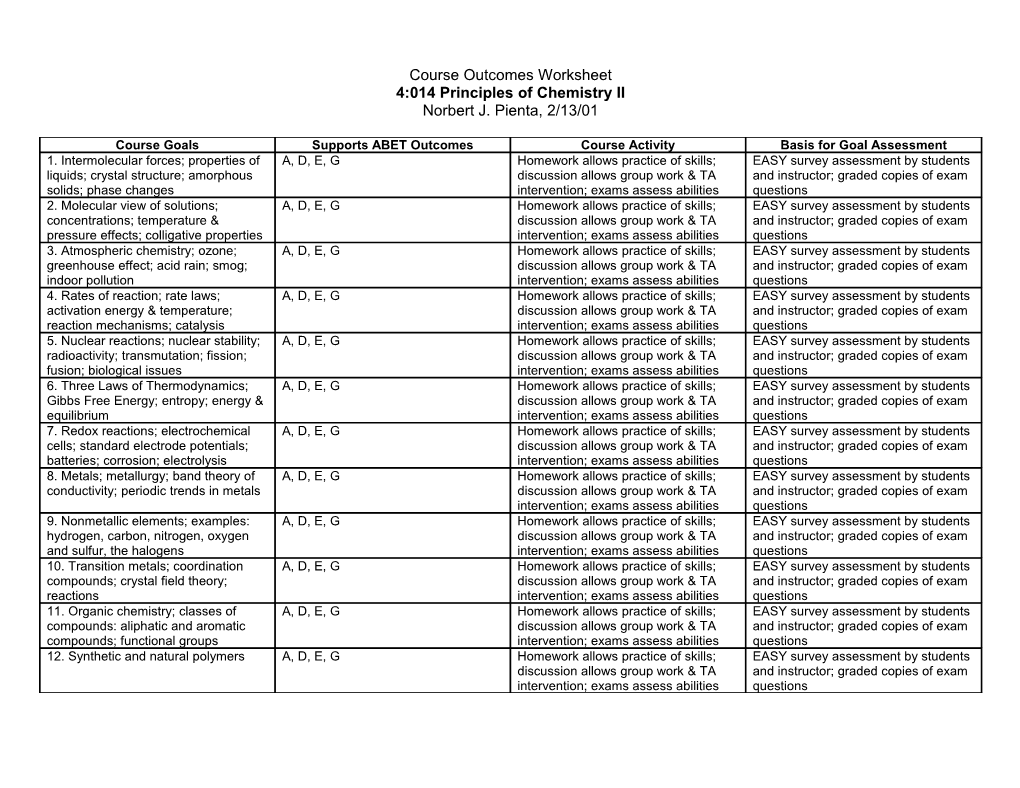
Course Goals Supports ABET Outcomes Course Activity Basis for Goal Assessment 1. Intermolecular forces; properties of A, D, E, G Homework allows practice of skills; EASY survey assessment by students liquids; crystal structure; amorphous discussion allows group work & TA and instructor; graded copies of exam solids; phase changes intervention; exams assess abilities questions 2. Molecular view of solutions; A, D, E, G Homework allows practice of skills; EASY survey assessment by students concentrations; temperature & discussion allows group work & TA and instructor; graded copies of exam pressure effects; colligative properties intervention; exams assess abilities questions 3. Atmospheric chemistry; ozone; A, D, E, G Homework allows practice of skills; EASY survey assessment by students greenhouse effect; acid rain; smog; discussion allows group work & TA and instructor; graded copies of exam indoor pollution intervention; exams assess abilities questions 4. Rates of reaction; rate laws; A, D, E, G Homework allows practice of skills; EASY survey assessment by students activation energy & temperature; discussion allows group work & TA and instructor; graded copies of exam reaction mechanisms; catalysis intervention; exams assess abilities questions 5. Nuclear reactions; nuclear stability; A, D, E, G Homework allows practice of skills; EASY survey assessment by students radioactivity; transmutation; fission; discussion allows group work & TA and instructor; graded copies of exam fusion; biological issues intervention; exams assess abilities questions 6. Three Laws of Thermodynamics; A, D, E, G Homework allows practice of skills; EASY survey assessment by students Gibbs Free Energy; entropy; energy & discussion allows group work & TA and instructor; graded copies of exam equilibrium intervention; exams assess abilities questions 7. Redox reactions; electrochemical A, D, E, G Homework allows practice of skills; EASY survey assessment by students cells; standard electrode potentials; discussion allows group work & TA and instructor; graded copies of exam batteries; corrosion; electrolysis intervention; exams assess abilities questions 8. Metals; metallurgy; band theory of A, D, E, G Homework allows practice of skills; EASY survey assessment by students conductivity; periodic trends in metals discussion allows group work & TA and instructor; graded copies of exam intervention; exams assess abilities questions 9. Nonmetallic elements; examples: A, D, E, G Homework allows practice of skills; EASY survey assessment by students hydrogen, carbon, nitrogen, oxygen discussion allows group work & TA and instructor; graded copies of exam and sulfur, the halogens intervention; exams assess abilities questions 10. Transition metals; coordination A, D, E, G Homework allows practice of skills; EASY survey assessment by students compounds; crystal field theory; discussion allows group work & TA and instructor; graded copies of exam reactions intervention; exams assess abilities questions 11. Organic chemistry; classes of A, D, E, G Homework allows practice of skills; EASY survey assessment by students compounds: aliphatic and aromatic discussion allows group work & TA and instructor; graded copies of exam compounds; functional groups intervention; exams assess abilities questions 12. Synthetic and natural polymers A, D, E, G Homework allows practice of skills; EASY survey assessment by students discussion allows group work & TA and instructor; graded copies of exam intervention; exams assess abilities questions EASY Assessment Questions 4:014 Principles of Chemistry II Norbert J. Pienta, 2/13/01
Question # EASY Assessment Statement 1a I understand the concepts of: intermolecular forces; properties of liquids; crystal structure; amorphous solids; phase changes 1b I can perform numerical problems involving dimensions of crystals 2a I understand the concepts of: molecular view of solutions; concentrations; temperature & pressure effects; colligative properties 2b I can perform numerical problems involving concentrations and colligative properties of solutions. 3 I understand the concepts of: atmospheric chemistry; ozone; greenhouse effect; acid rain; smog; indoor pollution 4a I understand the concepts of: rates of reaction; rate laws; activation energy & temperature; reaction mechanisms; catalysis 4b I can perform numerical problems involving rate expressions, integrated rate laws, and activation energy. 5a I understand the concepts of: nuclear reactions; nuclear stability; radioactivity; transmutation; fission; fusion; biological issues 5b I can perform numerical problems involving nuclear decay and half-lives. 6a I understand the concepts of: the Three Laws of Thermodynamics; Gibbs free energy; entropy; energy & equilibrium 6b I can perform numerical problems involving Gibbs free energy, entropy, and equilibrium. 7a I understand the concepts of: redox reactions; electrochemical cells; standard electrode potentials; batteries; corrosion; electrolysis 7b I can perform numerical problems involving electrode potentials. 8 I understand the concepts of: metals; metallurgy; band theory of conductivity; periodic trends in metals 9 I understand the concepts of: nonmetallic elements; examples: hydrogen, carbon, nitrogen, oxygen and sulfur, the halogens 10 I understand the concepts of: transition metals; coordination compounds; crystal field theory; reactions 11 I understand the concepts of: organic chemistry; classes of compounds: aliphatic and aromatic compounds; functional groups 12 I understand the concepts of: synthetic and natural polymers