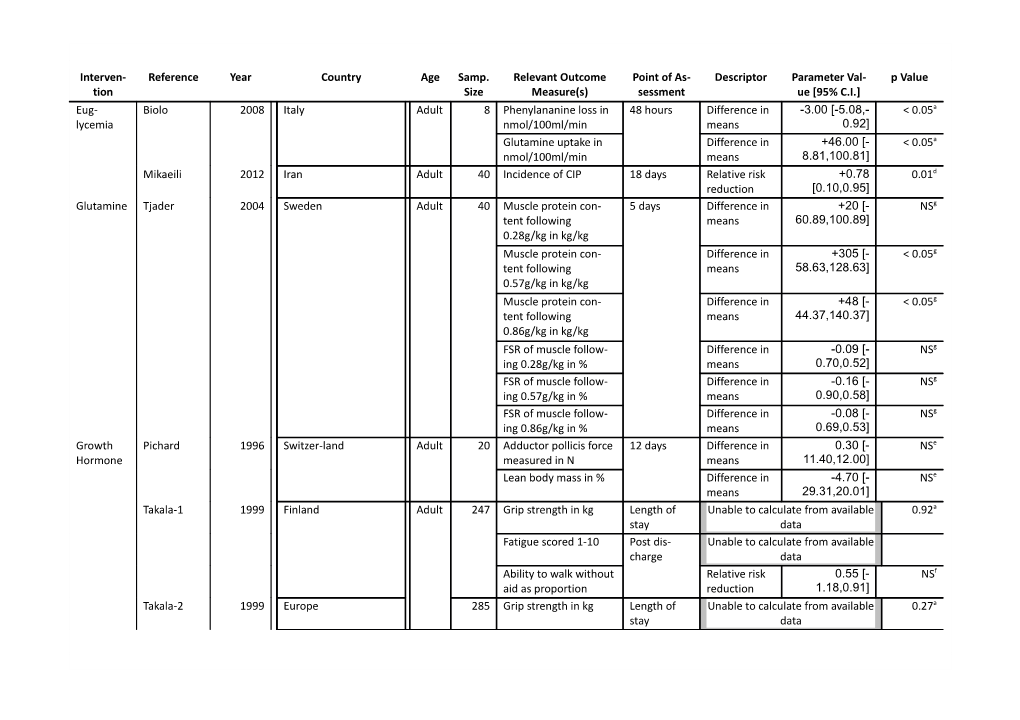
Interven- Reference Year Country Age Samp. Relevant Outcome Point of As- Descriptor Parameter Val- p Value tion Size Measure(s) sessment ue [95% C.I.] Eug- Biolo 2008 Italy Adult 8 Phenylananine loss in 48 hours Difference in -3.00 [-5.08,- < 0.05a lycemia nmol/100ml/min means 0.92] Glutamine uptake in Difference in +46.00 [- < 0.05a nmol/100ml/min means 8.81,100.81] Mikaeili 2012 Iran Adult 40 Incidence of CIP 18 days Relative risk +0.78 0.01d reduction [0.10,0.95] Glutamine Tjader 2004 Sweden Adult 40 Muscle protein con- 5 days Difference in +20 [- NSg tent following means 60.89,100.89] 0.28g/kg in kg/kg Muscle protein con- Difference in +305 [- < 0.05g tent following means 58.63,128.63] 0.57g/kg in kg/kg Muscle protein con- Difference in +48 [- < 0.05g tent following means 44.37,140.37] 0.86g/kg in kg/kg FSR of muscle follow- Difference in -0.09 [- NSg ing 0.28g/kg in % means 0.70,0.52] FSR of muscle follow- Difference in -0.16 [- NSg ing 0.57g/kg in % means 0.90,0.58] FSR of muscle follow- Difference in -0.08 [- NSg ing 0.86g/kg in % means 0.69,0.53] Growth Pichard 1996 Switzer-land Adult 20 Adductor pollicis force 12 days Difference in 0.30 [- NSe Hormone measured in N means 11.40,12.00] Lean body mass in % Difference in -4.70 [- NSe means 29.31,20.01] Takala-1 1999 Finland Adult 247 Grip strength in kg Length of Unable to calculate from available 0.92a stay data Fatigue scored 1-10 Post dis- Unable to calculate from available charge data Ability to walk without Relative risk 0.55 [- NSf aid as proportion reduction 1.18,0.91] Takala-2 1999 Europe 285 Grip strength in kg Length of Unable to calculate from available 0.27a stay data Interven- Reference Year Country Age Samp. Relevant Outcome Point of As- Descriptor Parameter Val- p Value tion Size Measure(s) sessment ue [95% C.I.] Fatigue scored 1-10 Post dis- Unable to calculate from available charge data Ability to walk unaided Relative risk 0.09 [- NSf as proportion reduction 1.09,0.61] Gamrin 2000 Sweden Adult 20 Muscle protein con- 4 days Difference in +207 [- < 0.05c tent in % means 26.92,440.92] FSR of muscle in % Difference in +33.00 [- 0.01c means 1.34,67.34] Im- Brunner 2012 Austria Adult 38 Novel CIPNM score Day 14 Difference in -0.29 [- NSd munoglob- means 1.83,1.25] ulin Quantitative CIP score Difference in -0.10 [- NSd means 1.26,1.06] Semiquantitative Difference in -0.20 [- NSd histopathological CIM means 9.89,9.50] score Oxan- Demling 2003 USA Adult 45 Weight gain in kg 4 weeks Difference in 3.00 < 0.05b drolone means [1.49,4.51] Lean body mass in % Discharge Difference in +5.00 < 0.05b from hospi- means [2.87,7.13]] tal Propra- Herndon 2001 USA Ped 25 Net balance of protein 2 weeks Difference in -0.08 [-0.12,- 0.001c nolol synthesis in means 0.03] µmol/100ml/min Lean body mass in % Difference in +5.60 0.01c means [1.53,9.67]
Supplementary Digital Content - Table 1: PICOS table of studies included in primary analysis a. Wilcoxon matched pair test b. Scheff test c. Student t-test d. Fisher’s exact test e. Mann-Whitney U test f. Chi-squared test g. ANOVA
Abbreviations:
ARDS - acute respiratory distress syndrome; CABG - coronary artery bypass grafting; CIM - critical illness polymyopathy; CIP - critical illness polyneuropathy; EP - electrophysiologi- cal; FSR - fractional synthetic rate; FT - full text; ICU - intensive care unit; LOS - length of stay; LBM - lean body mass; NS - non-significant; ped - pediatric; PN - parenteral nutrition; rehab. - rehabilitation; rhGH - growth hormone; sig. - significant; samp. - sample.