Chemistry 12 Experiment 20-D
Name ______Name ______Date ______Due Date ______Correct and Hand In By ______Chemistry 12 Experiment 20-D Hydrolysis 10
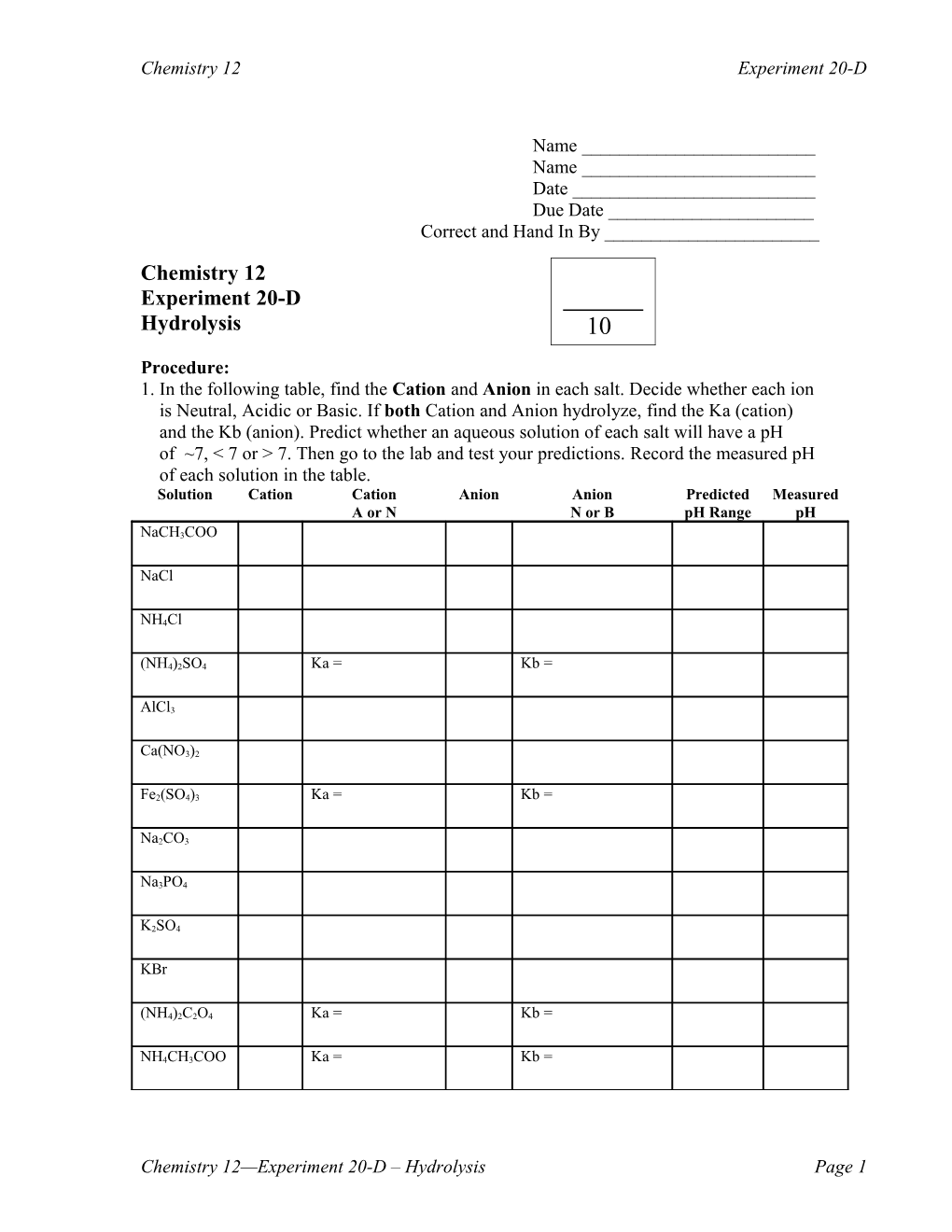
Procedure: 1. In the following table, find the Cation and Anion in each salt. Decide whether each ion is Neutral, Acidic or Basic. If both Cation and Anion hydrolyze, find the Ka (cation) and the Kb (anion). Predict whether an aqueous solution of each salt will have a pH of ~7, < 7 or > 7. Then go to the lab and test your predictions. Record the measured pH of each solution in the table. Solution Cation Cation Anion Anion Predicted Measured A or N N or B pH Range pH
NaCH3COO
NaCl
NH4Cl
(NH4)2SO4 Ka = Kb =
AlCl3
Ca(NO3)2
Fe2(SO4)3 Ka = Kb =
Na2CO3
Na3PO4
K2SO4
KBr
(NH4)2C2O4 Ka = Kb =
NH4CH3COO Ka = Kb =
Chemistry 12—Experiment 20-D – Hydrolysis Page 1 Chemistry 12 Experiment 20-D
2 For the following solutions containing salts of amphiprotic anions, find the amphiprotic anion, it’s Ka and it’s Kb and predict it’s approximate pH range (~7, < 7 or > 7). Then go into the lab and test your predictions. The first one has been done as an example. Record the measured pH’s on the following table:
Solution Amphiprotic Ka Kb Predicted Measured Anion pH Range pH 2- -13 -14 -7 K2HPO4 HPO4 2.2 x 10 1.0 x 10 = 1.6 x 10 > 7 6.2 x 10-8
KH2PO4
NaHCO3
KHSO4
NaHSO3
Questions:
1. Write a balanced Net-Ionic Hydrolysis Equation for each Cation which hydrolyzed in Part 1 of the lab. (Don’t repeat any)
______
______
______
2. Write a balanced Net-Ionic Hydrolysis Equation for each Anion which hydrolyzed in Part 1 of the lab. (Don’t repeat any)
______
______
______
______
______
Chemistry 12—Experiment 20-D – Hydrolysis Page 2 Chemistry 12 Experiment 20-D
3. Write a balanced Net-Ionic Hydrolysis Equation showing the predominant hydrolysis reaction for each Amphiprotic Anion in Part 2. (Be careful to observe whether the predominant hydrolysis is acidic or basic. See the table in procedure 2.)
Amphiprotic Anion Acid or Base Hydrolysis Equation Hydrolysis 2- HPO4
- H2PO4
- HCO3
- HSO4
- HSO3
4. Two types of fertilizers are KNO3 and (NH4)2SO4. Fill in the following table:
Solution Cation Cation Anion Anion Predicted A or N N or B pH Range
KNO3
(NH4)2SO4 Ka = Kb =
Which fertilizer is more acidic? ______
5. Write the net-ionic equation showing the dissociation of sodium phosphate:
______
3- Write the net-ionic equation showing the hydrolysis of phosphate (PO4 ) :
______
Explain why a compound in stores called Tri Sodium Phosphate (TSP) is used as a cleaner for walls and floors. (The proper name is Sodium Phosphate or Na3PO4.)
Chemistry 12—Experiment 20-D – Hydrolysis Page 3