UK NEQAS for H&I – SCHEME 3 – HLA ANTIBODY SPECIFICITY ANALYSIS METHODOLOGY QUESTIONNAIRE
Please complete the following information for the methods used by your laboratory for Scheme 3. If your laboratory makes any changes to these methods during the assessment year, please resubmit this form with the changes. The results of this questionnaire will be collated and made available on the UK NEQAS for H&I website.
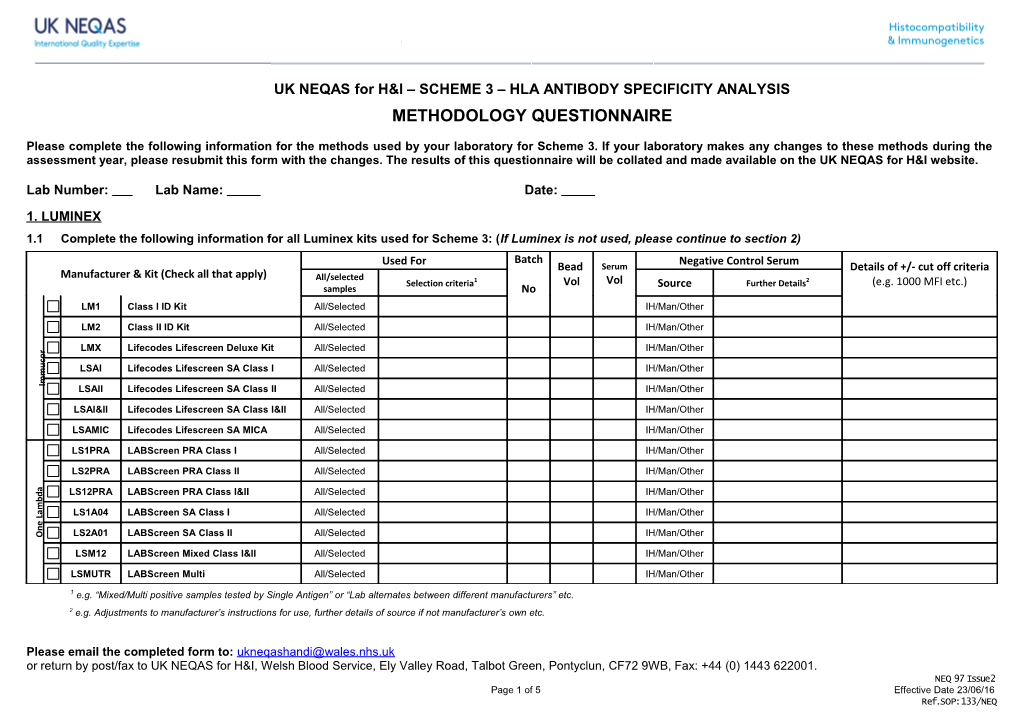
Lab Number: Lab Name: Date: 1. LUMINEX 1.1 Complete the following information for all Luminex kits used for Scheme 3: (If Luminex is not used, please continue to section 2) Used For Batch Negative Control Serum Bead Serum Details of +/- cut off criteria Manufacturer & Kit (Check all that apply) All/selected Selection criteria1 Vol Vol Source Further Details2 (e.g. 1000 MFI etc.) samples No LM1 Class I ID Kit All/Selected IH/Man/Other
LM2 Class II ID Kit All/Selected IH/Man/Other
LMX Lifecodes Lifescreen Deluxe Kit All/Selected IH/Man/Other r o c u LSAI Lifecodes Lifescreen SA Class I All/Selected IH/Man/Other m m I LSAII Lifecodes Lifescreen SA Class II All/Selected IH/Man/Other
LSAI&II Lifecodes Lifescreen SA Class I&II All/Selected IH/Man/Other
LSAMIC Lifecodes Lifescreen SA MICA All/Selected IH/Man/Other
LS1PRA LABScreen PRA Class I All/Selected IH/Man/Other
LS2PRA LABScreen PRA Class II All/Selected IH/Man/Other a
d LS12PRA LABScreen PRA Class I&II All/Selected IH/Man/Other b m
a LS1A04 LABScreen SA Class I All/Selected IH/Man/Other L
e n
O LS2A01 LABScreen SA Class II All/Selected IH/Man/Other
LSM12 LABScreen Mixed Class I&II All/Selected IH/Man/Other
LSMUTR LABScreen Multi All/Selected IH/Man/Other
1 e.g. “Mixed/Multi positive samples tested by Single Antigen” or “Lab alternates between different manufacturers” etc. 2 e.g. Adjustments to manufacturer’s instructions for use, further details of source if not manufacturer’s own etc.
Please email the completed form to: [email protected] or return by post/fax to UK NEQAS for H&I, Welsh Blood Service, Ely Valley Road, Talbot Green, Pontyclun, CF72 9WB, Fax: +44 (0) 1443 622001. NEQ 97 Issue 2 Page 1 of 5 Effective Date 23/06/16 Ref. SOP: 133/NEQ UK NEQAS for H&I SCHEME 3 - HLA ANTIBODY
UK NEQAS for H&I SCHEME 3 - HLA ANTIBODY SPECIFICITY ANALYSIS
Lab Number: Lab Name:
1.2 Positive Control Serum Does your laboratory use positive control sera for Luminex testing? Yes/No If “Yes”, please provide further details below (If “No” go to part 1.3) Source (e.g. sensitised patient sera etc.):
If “No” please specify/further details
Is the positive control sera included in Yes No every run?
If “No” please specify/further details
Is the same positive control serum Yes No used for all Luminex kits?
1.3 Are serum samples stored prior to testing? Yes/No If yes, please specify the storage conditions: Frozen Fridge Other (please specify): (specify temp): (specify temp):
Approximately how long are the samples stored prior to testing? <24 hours 24-48 hours >48 hours
1.4 Serum Treatment Please indicate all methods used in your laboratory for this Scheme: N/A – No sera are treated Used for: Product: Adsorb Bead:Serum ratio: Adsorption Beads All/Selected Samples Out/SeraClean Used for: Time & Temperature: Heat inactivation All/Selected Samples Used for: Added to: Sera/Wash Conc of stock solution: All/Selected Samples Buffer EDTA Volume added: µl EDTA in µl sera/wash buffer
Is there a minimum EDTA Yes/No: If yes, specify: treatment incubation time?
Are the samples stored, Yes/No If yes, please provide further details: following EDTA treatment, prior to testing?
Please email the completed form to: [email protected] or return by post/fax to UK NEQAS for H&I, Welsh Blood Service, Ely Valley Road, Talbot Green, Pontyclun, CF72 9WB, Fax: +44 (0) 1443 622001. NEQ 97 Issue 2 Page 2 of 5 Effective Date 23/06/16 Ref. SOP: 133/NEQ UK NEQAS for H&I SCHEME 3 - HLA ANTIBODY
UK NEQAS for H&I SCHEME 3 - HLA ANTIBODY SPECIFICITY ANALYSIS
Lab Number: Lab Name:
Serum Treatment Contd. Used for: All/Selected Diluted in (e.g. wash buffer): Dilutions used (e.g. 1 in Dilution Samples 10): Other (specify) Used for: All/Selected Samples
If you have indicated that only selected samples are treated with any of the above, please specify the selection criteria (e.g. samples with High Background etc.):
1.5 Incubation times: As per manufacturer’s Other (please specify): instructions
1.6 Wash step details: Other (please specify): Wash Medium: Manufacturer supplied
Is anything added?
No EDTA (see 1.4 above) Other (please specify):
First wash step: Number of washes: Wash volume per sample/well (µl):
Second wash step (if applicable): Number of washes: Wash volume per sample/well (µl):
Wash Buffer removed by: Centrifuge, Vacuum Manifold Other (please specify): ‘flick and blot’
1.7 Data Analysis
Which Software is used to analyse the data?
What is the minimum ‘bead count’ accepted by your laboratory (e.g. 100)?
What is the minimum ‘Positive Control’ bead MFI value accepted by your laboratory (e.g. 5,000)?
What is the criteria for classifying a sample as having ‘High Background'?
Please email the completed form to: [email protected] or return by post/fax to UK NEQAS for H&I, Welsh Blood Service, Ely Valley Road, Talbot Green, Pontyclun, CF72 9WB, Fax: +44 (0) 1443 622001. NEQ 97 Issue 2 Page 3 of 5 Effective Date 23/06/16 Ref. SOP: 133/NEQ UK NEQAS for H&I SCHEME 3 - HLA ANTIBODY
UK NEQAS for H&I SCHEME 3 - HLA ANTIBODY SPECIFICITY ANALYSIS
Lab Number: Lab Name:
2. COMPLEMENT DEPENDENT CYTOTOXICITY (CDC)
Do you use CDC to detect IgG HLA antibodies in Scheme 3? Yes/No (If “No” please continue to section 3) Technique: e.g. NIH, extended NIH, AHG, etc.: Comments:
Cell source: PBL T-cells B-cells B-cell lines CLL cells Other (specify)
Manufacturer: Do you use frozen If “Yes”, Prepared in-house Commercial Yes/No cell trays? are they:
Selected Random Mixture Panel size: Panel composition:
Complement Prepared in-house Commercial Manufacturer: source: Volume added (µl per Cells: Sera: Complement: DTT: well): Incubation times: Pre complement addition: Post complement addition: (in minutes) How do you Ethidium Bromide(+Acridine Orange ) Propidium Iodide(+Acridine Orange) Fluoroquench Eosin visualise cell death: Analysis Manual Computerised procedures: If computerised is In-house Commercial Manufacturer: software produced:
Please email the completed form to: [email protected] or return by post/fax to UK NEQAS for H&I, Welsh Blood Service, Ely Valley Road, Talbot Green, Pontyclun, CF72 9WB, Fax: +44 (0) 1443 622001. NEQ 97 Issue 2 Page 4 of 5 Effective Date 23/06/16 Ref. SOP: 133/NEQ UK NEQAS for H&I SCHEME 3 - HLA ANTIBODY
UK NEQAS for H&I SCHEME 3 - HLA ANTIBODY SPECIFICITY ANALYSIS Lab Number: Lab Name:
3. FLOW CYTOMETRY
Flow Cytometer: Individual cell Pooled cell Number
preparations: preparations: in pool: Cell source: PBL T-cells B-cells B-cell lines CLL cells One Lambda FlowPRATM Other (specify):
CONJUGATE USED MANUFACTURER
SOFTWARE USED MANUFACTURER
4. ELISA
KIT USED BATCH NO MANUFACTURER
OTHER TECHNIQUE(S) (please give brief details)
COMMENTS:
Please email the completed form to: [email protected] or return by post/fax to UK NEQAS for H&I, Welsh Blood Service, Ely Valley Road, Talbot Green, Pontyclun, CF72 9WB, Fax: +44 (0) 1443 622001. NEQ 97 Issue 2 Page 5 of 5 Effective Date 23/06/16 Ref. SOP: 133/NEQ