Chemistry Activity Name: Periodic Trends Period:
The periodic table not only provides us with atomic numbers of elements and masses of elements, but it also provides us with a tool to determine general trends in chemical and physical properties.
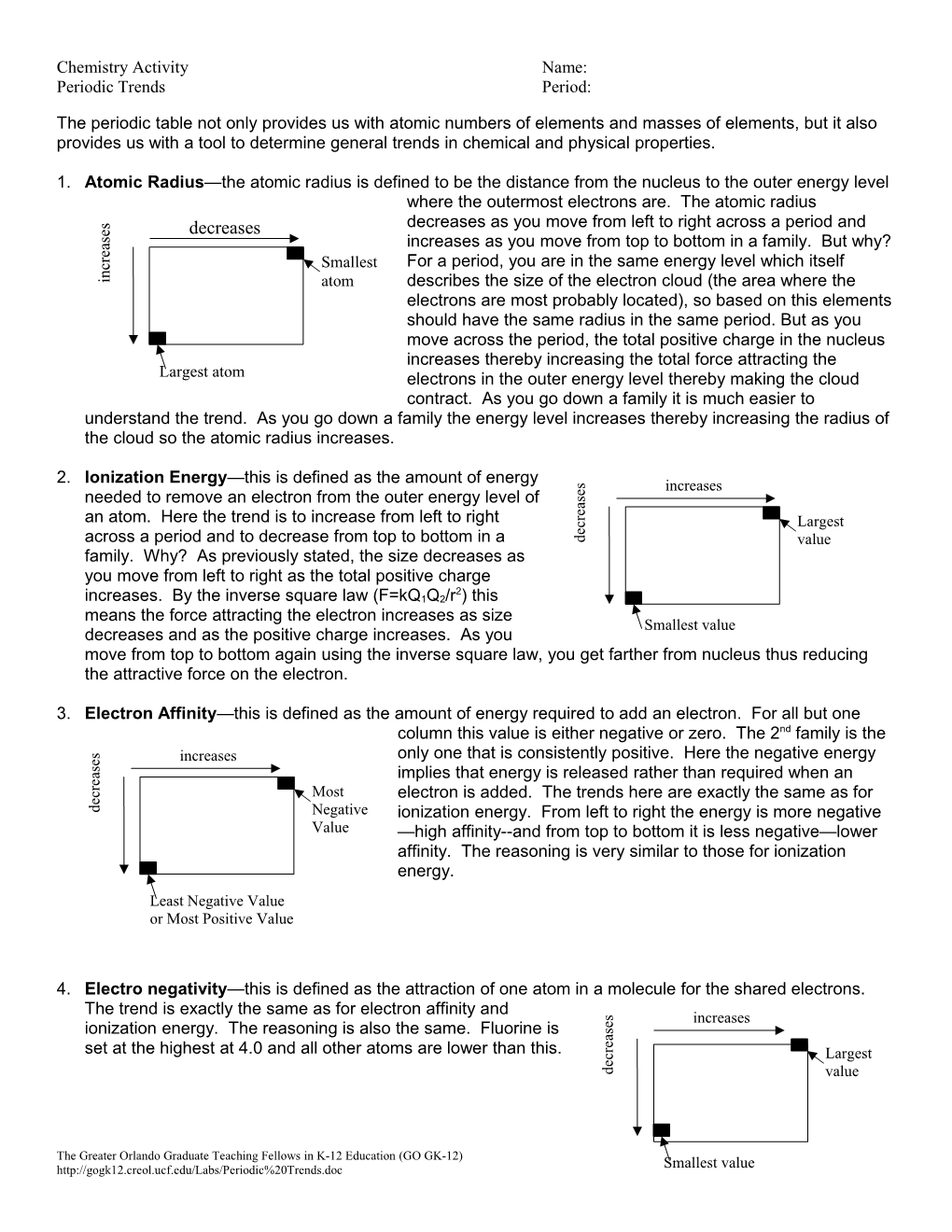
1. Atomic Radius—the atomic radius is defined to be the distance from the nucleus to the outer energy level where the outermost electrons are. The atomic radius
s decreases as you move from left to right across a period and
e decreases s
a increases as you move from top to bottom in a family. But why? e r For a period, you are in the same energy level which itself c Smallest n i atom describes the size of the electron cloud (the area where the electrons are most probably located), so based on this elements should have the same radius in the same period. But as you increase move across the period, the total positive charge in the nucleus increases thereby increasing the total force attracting the Largest atom electrons in the outer energy level thereby making the cloud contract. As you go down a family it is much easier to understand the trend. As you go down a family the energy level increases thereby increasing the radius of the cloud so the atomic radius increases.
2. Ionization Energy—this is defined as the amount of energy s increases e
needed to remove an electron from the outer energy level of s a e
an atom. Here the trend is to increase from left to right r
c Largest e
across a period and to decrease from top to bottom in a d value family. Why? As previously stated, the size decreases as you move from left to right as the total positive charge 2 increases. By the inverse square law (F=kQ1Q2/r ) this means the force attracting the electron increases as size Smallest value decreases and as the positive charge increases. As you move from top to bottom again using the inverse square law, you get farther from nucleus thus reducing the attractive force on the electron.
3. Electron Affinity—this is defined as the amount of energy required to add an electron. For all but one column this value is either negative or zero. The 2nd family is the
s increases only one that is consistently positive. Here the negative energy e s
a implies that energy is released rather than required when an e r
c Most electron is added. The trends here are exactly the same as for e d Negative ionization energy. From left to right the energy is more negative Value —high affinity--and from top to bottom it is less negative—lower affinity. The reasoning is very similar to those for ionization energy. Least Negative Value or Most Positive Value
4. Electro negativity—this is defined as the attraction of one atom in a molecule for the shared electrons. The trend is exactly the same as for electron affinity and s increases e
ionization energy. The reasoning is also the same. Fluorine is s a e
set at the highest at 4.0 and all other atoms are lower than this. r
c Largest e
d value
The Greater Orlando Graduate Teaching Fellows in K-12 Education (GO GK-12) http://gogk12.creol.ucf.edu/Labs/Periodic%20Trends.doc Smallest value Chemistry Activity Name: Periodic Trends Period:
5. Reactivity—this is defined as how readily an atom reacts. For metals this is based on ionization energy where the most reactive metals are those with the lowest ionization energies. For nonmetals this is based on electron affinity where the most negative electron affinity is the most reactive. This has to do with the fact that metals form positive ions (lose electrons) and nonmetals for negative ions (gains electrons).
Materials graph paper activity series for metals and nonmetals (found in any chemistry text or on the internet)
Description For the following data, plot the atomic number on the x-axis and the property on the y-axis. Each property should be graphed on a separate sheet of graph paper.
Element Atomic Atomic Radius 1st Ionization Electron Affinity Electro Number (pm) Energy (kJ/mol) (kJ/mol) negativity (Pauling Units) H 1 53 1312 -73 2.1 He 2 31 2372 0 NA Li 3 167 520 -60 1.0 Be 4 112 899 240 1.5 B 5 87 801 -27 2.0 C 6 67 1086 -122 2.5 N 7 56 1402 0 3.0 O 8 48 1314 -141 3.5 F 9 42 1681 -328 4.0 Ne 10 38 2081 0 NA Na 11 190 496 -53 0.9 Mg 12 145 738 230 1.2 Al 13 118 578 -44 1.5 Si 14 111 787 -134 1.8 P 15 98 1012 -72 2.1 S 16 88 1000 -200 2.5 Cl 17 79 1251 -349 3.0 Ar 18 71 1521 0 NA
The Greater Orlando Graduate Teaching Fellows in K-12 Education (GO GK-12) http://gogk12.creol.ucf.edu/Labs/Periodic%20Trends.doc Chemistry Activity Name: Periodic Trends Period:
Analysis 1. Based on your graphs, what is the trend in atomic radius across a period? down a family?
2. Based on your graphs, what is the trend in ionization energy across a period? down a family?
3. Based on your graphs, what is the trend in electron affinity across a period? down a family?
4. Based on your graphs, what is the trend in electro negativity across a period? down a family?
5. Using an activity series, what can you deduce about the relationship between ionization energy and reactivity of metals?
6. Using an activity series, what can you deduce about the relationship between electron affinity and reactivity of nonmetals?
7. Explain in 3-4 paragraphs the organization and usefulness of the modern periodic table based on what you have learned in class and what you have learned from this activity. (See teacher notes).
The Greater Orlando Graduate Teaching Fellows in K-12 Education (GO GK-12) http://gogk12.creol.ucf.edu/Labs/Periodic%20Trends.doc