Unit 8 Section 1: Stoichiometry Obj. 1
What do coefficients mean?
The ______of the balanced equation tell how many moles or particles of each substance is used in the reaction. A Mole ratio is a ______that relates 2 substances in moles. You must use a balanced chemical equation to create it. What are all the possible mole ratios of:
2 H2 + O2 2 H2O
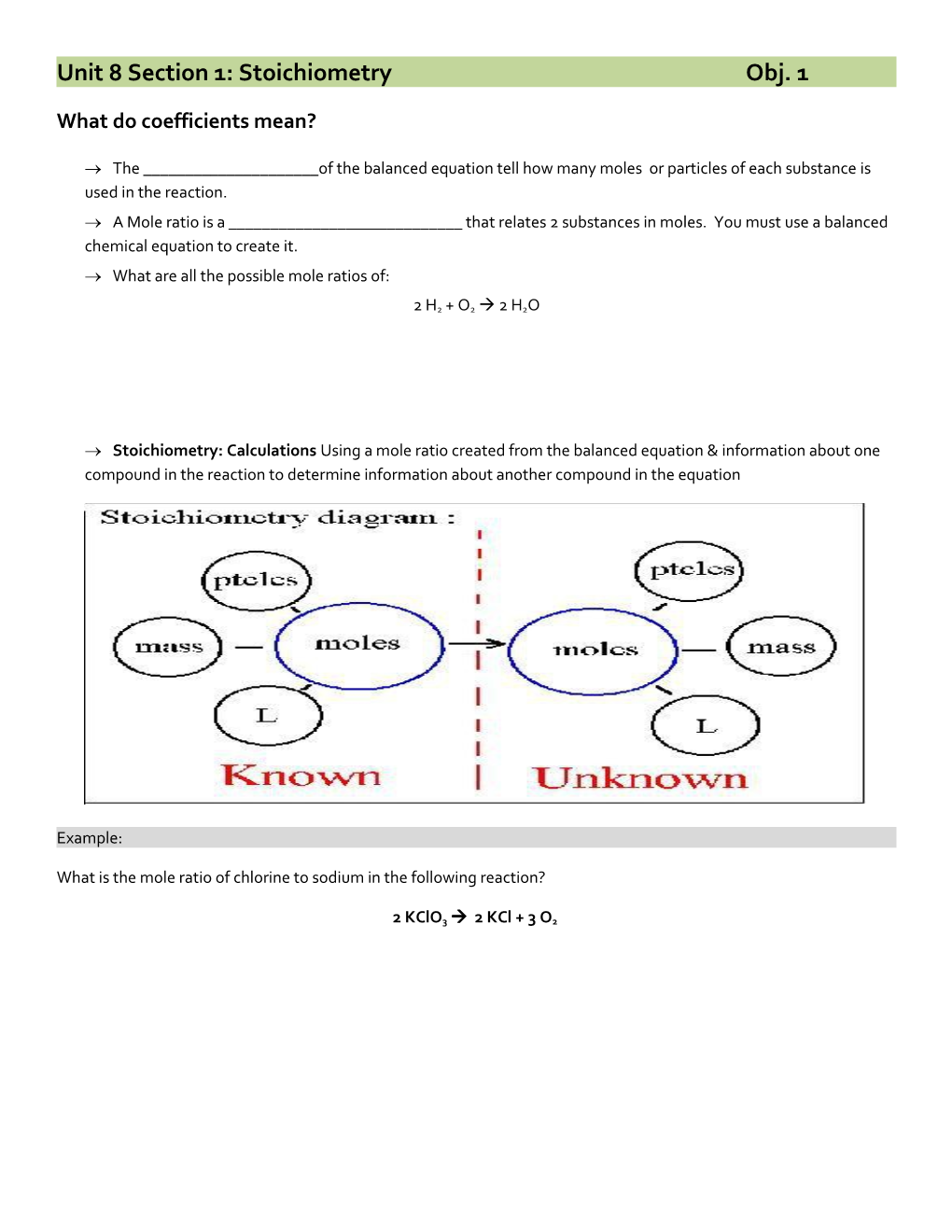
Stoichiometry: Calculations Using a mole ratio created from the balanced equation & information about one compound in the reaction to determine information about another compound in the equation
Example:
What is the mole ratio of chlorine to sodium in the following reaction?
2 KClO3 à 2 KCl + 3 O2 General Stoichiometry Calculations
ALMOST ALL STOICHIOMETRIC PROBLEMS CAN BE SOLVED IN 4 SIMPLE STEPS
1. Balance the equation. 2. Convert units of a given substance to moles. 3. Using the mole ratio, calculate the moles of substance yielded by the reaction. 4. Convert moles of wanted substance to desired units.
Mole and Mole : 1 step Problem USE ONLY THE MOLE RATIO
1. If 4.2 moles of H2 reacts completely with O2, how many moles of O2 are needed?
2 H2 + O2 à 2 H2O
2. Given the BALANCED EQUATION: 2KNO3 2KNO2 + O2, how many moles of oxygen are produced by the decomposition of 0.67 moles of potassium nitrate, KNO3?
Moles and Mass : 2 step Problem
We can’t measure moles in the lab. We can only measure grams.
Molar Mass (grams) = 1 mole of a compound
Mole-Mass (2 step problem)
1. How many grams of AgCl will be precipitated if 0.45 mole AgNO3 is reacted as follows:
2 AgNO3 + CaCl2 2 AgCl + Ca(NO3)2 2. Given the BALANCED EQUATION: N2 + 3H2 2NH3
How many moles of ammonia, NH3 are produced from 4.42 g of hydrogen gas, H2? Stoichiometry & Gases: Obj. 3
Recall: Molar Volume of a Gas – at STP, 1 mole of any gas = 22.4 liters
1. If you need to react 1.5 g of zinc completely, what volume of hydrogen gas will be produced at STP?
2 HCl (aq) + Zn (s) ZnCl2 (aq) + H2 (g)
2. Given the balanced equation: C3H8 + 5O2 → 3CO2 + 4H2O
How many moles of water will be produced from the complete combustion of 7.3 L of oxygen gas? Assume STP
Keeping all these MOLE equalities straight! To Go Between… Use the Equality…
Particles and Moles 1 mole = 6.02 x 1023 atoms, molecules or FU
Grams and Moles 1 mole = molar mass (grams)
Moles and liters of a gas at STP 1 mole =22.4 liters at STP
Coefficient ratio (mole ratio) from balanced 2 different chemicals in a reaction equation
Let’s Practice: You Try!
1. Given the UNBALANCED EQUATION: __MgCO3 __MgO + __CO2, how many L of carbon
dioxide gas (CO2)are produced from the reaction of 15 grams of MgCO3? Assume STP !
Unit 7 Section 2: Obj. 2 cont.
Percent Yield
A “Yield” is a product An “Actual Yield” (A): the actual amount of product produced in the lab A “Theoretical Yield” (T) : the amount of product you should produce if nothing went wrong; use the balanced chemical equation to calculate it A “Percent Yield”: ratio of the actual yield compared to the theoretical yield
% Yield = Let’s Practice: Let’s take this one in steps 1a. 4.20 moles of H2 reacts completely with O2, how many grams of H2O are produced (otherwise known as the Theoretical Yield)? 2H2 + O2 2 H2O
Theoretical yield of water = ______g
1b What is the percent yield if 60.0 grams of H2O are actually produced?
2.You have precipitated 8.5 g of Ba(OH)2. If you started with 4.57 grams NaOH, what is the percent yield? 2 NaOH + BaCl2 Ba(OH)2 + 2 NaCl
Hint: Identify the actual yield. Calculate the theoretical yield of Ba(OH)2 using the reactant amount
.
Unit 7 Section 3: Extension
Limiting Reagent Every day Example: …… if we had 10 servings of both peanut butter and jelly, but only 4 slices of bread we could only make two complete peanut butter and jelly sandwiches. In this example the four slices of bread is the limiting reagent and so determines how many sandwiches (product) could be produced. The servings of peanut butter and jelly could be described as excess reagents. ( taken from C. Ferwerda’ site)
Chemistry Example Recipe: To produce ammonia Calls for:
3H2 + 1N2 2NH3 3 mol H2 reacts with 1 mol N2 to make 2 mols NH3 What would happen if 3 mol H2 reacted with 2 mol N2?
Given:Initial 3 mol H2 2 mol N2 0 mol NH3
Left over: Final 0 mol H2 1 mol N2 2 mol NH3 What is all used up? ______ Limiting Reagent – the substances that limits and thus determines the amount of product that can form; it gets used up first
What is left over? ______ Excess Reagent – the substance that is left over; more than enough to react with limiting reagent
How much product was produced? ______
Examples: 2Na + 1 Cl2 2NaCl A) What will occur when 6.70 mol Na reacts with 3.2 mol Cl2? Step 1 – Convert one of the reactants to the other
Step 2 – Is the amount you calculated: larger than the given amountin the problem? limiting reagent (…..Your need is greater than what you have been given…..) OR smaller than the given amount in the problem excess reagent (…….Your need is less than what you have been given……)
B) How much of the excess reagent do you have? *must use limiting reagent to convert amount of other reactant needed*
C) How much NaCl is produced? (in moles) *must use limiting reagent to determine amount of product produced
Alternate Approach! Take each reactant to the same product Compare amounts smaller amount will identify LR
Example: 2 Cu + S Cu2S What is the maximum number of grams of Cu2S that can be formed when 80.0g of Cu reacts with 25.0g of S? *Always begin in the units of mole*
You Try Problem CO +2H2 CH3OH a. If 500.0 mol CO react with 750.0 mol H2 what is LR?
b. How many moles of excess are left over?
c. How many moles of CH3OH are produced?