Supporting Information
Catalytic Performance Comparison of Shape-Dependent Nanocrystals
and Textured Ultra Thin Films of Pt4Cu Alloy in the Formic Acid Oxidation Process
L. Bromberg1, M. Fayette1, B. Martens1, Z. Luo2, Y. Wang1, D. Xu1, J. Zhang1, J. Fang1*, and N. Dimitrov1** 1) Department of Chemistry, State University of New York at Binghamton, PO Box 6000, Binghamton, New York 13902 (USA) 2) Microscopy and Imaging Center and Materials Science and Engineering Program, Texas A&M University, College Station, Texas 77843 Phone: +1 (607) 777-3752*; +1 (607) 777-4271** Fax: +1 (607) 777-4478 Email: [email protected];* [email protected]** URL: http://nano.binghamton.edu ; * http://chemiris.chem.binghamton.edu/DIMITROV/dimitrov.htm**
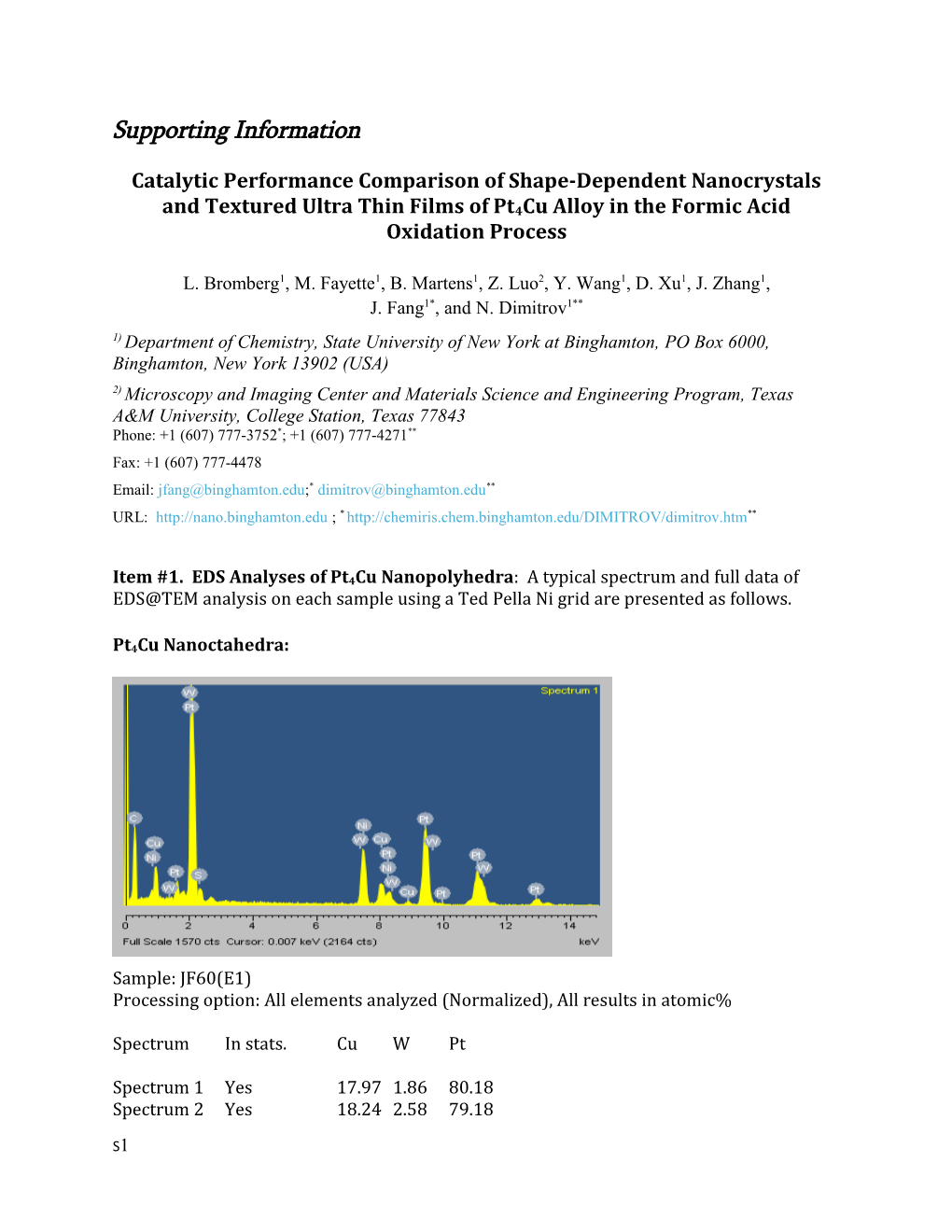
Item #1. EDS Analyses of Pt4Cu Nanopolyhedra: A typical spectrum and full data of EDS@TEM analysis on each sample using a Ted Pella Ni grid are presented as follows.
Pt4Cu Nanoctahedra:
Sample: JF60(E1) Processing option: All elements analyzed (Normalized), All results in atomic%
Spectrum In stats. Cu W Pt
Spectrum 1 Yes 17.97 1.86 80.18 Spectrum 2 Yes 18.24 2.58 79.18
S1 Spectrum 3 Yes 19.16 2.08 78.75 Spectrum 4 Yes 18.90 3.07 78.03 Spectrum 5 Yes 14.48 2.20 83.32
Mean 17.75 2.36 79.89 Std. deviation 1.89 0.47 2.07 Max. 19.16 3.07 83.32 Min. 14.48 1.86 78.03 ------
Pt4Cu Nanocubes:
Sample: JF31_2A4 Processing option: All elements analyzed (Normalized), All results in atomic%
Spectrum In stats. Cu Pt
Spectrum 1 Yes 11.26 88.74 Spectrum 2 Yes 15.25 84.75 Spectrum 3 Yes 17.06 82.94 Spectrum 4 Yes 16.80 83.20 Spectrum 5 Yes 14.20 85.80
Mean 14.91 85.09 Std. deviation 2.35 2.35 Max. 17.06 88.74 Min. 11.26 82.94
2 Item #2. H UPD CV curves registered throughout the long-term formic acid oxidation testing.
Item #3. Formic acid oxidation CV curves registered throughout the long-term formic acid oxidation testing.
Item #4. Durability of thin film and bulk Pt catalysts assessed in formic acid oxidation long-term testing.
S3 Item #5. Amount of Pt in Nano-octahedra (NO) and Nanocubes (NCs).
In order to determine the amount of catalyst present in the nanoparticles, it was assumed for the sake of calculation that the catalyst contained only platinum, and lastly that the particles did not overlap. The figure below presents a schematic of NO and NCs indicating the dimesions as reported in the main text of this article.
1.) Volume of the nanoparticles and number of Pt atoms per particle
2.) Surface area per particle and total number of particles in our experiment
The surface area of each particle type was calculated only for 5 sides (NCs) and 7 sides (NO) as one side must remain in contact with the substrate. In order to ascertain the amount of each particle, the ECASA for each type (5.6 cm2 for NC and 3.8 cm2 for NO) was used assuming that the H UPD layer fully coated the entire particle.
4 3.) Number of Pt monolayers (ML) per 1 cm2 electrochemically active surface area
With all the above information, we will now determine the amount of catalyst in terms of the number of monolayers (ML’s) in order to compare the amount of catalyst present in the nanoparticles to the thin films which are approximately 7-8 ML’s thick and that 1 ML of Pt is 1.5 x 1015 atoms/cm2 for the (111) face and 1.3 x 1015 atoms/cm2 for the (100) face.
Item #6. H UPD CV curves on single-crystalline Pt (hkl) electrodes.
S5