Lesson 18. Photosynthesis: Putting the Pieces Together
Objective: Reinforce the concept of photosynthesis from the research presentations.
EQ: What is involved in autotrophic nutrition?
Bridge: Have students record everything that they know about photosynthesis, both from prior knowledge and from the presentations last week. They can do this in pairs and as a narrative or as a diagram.
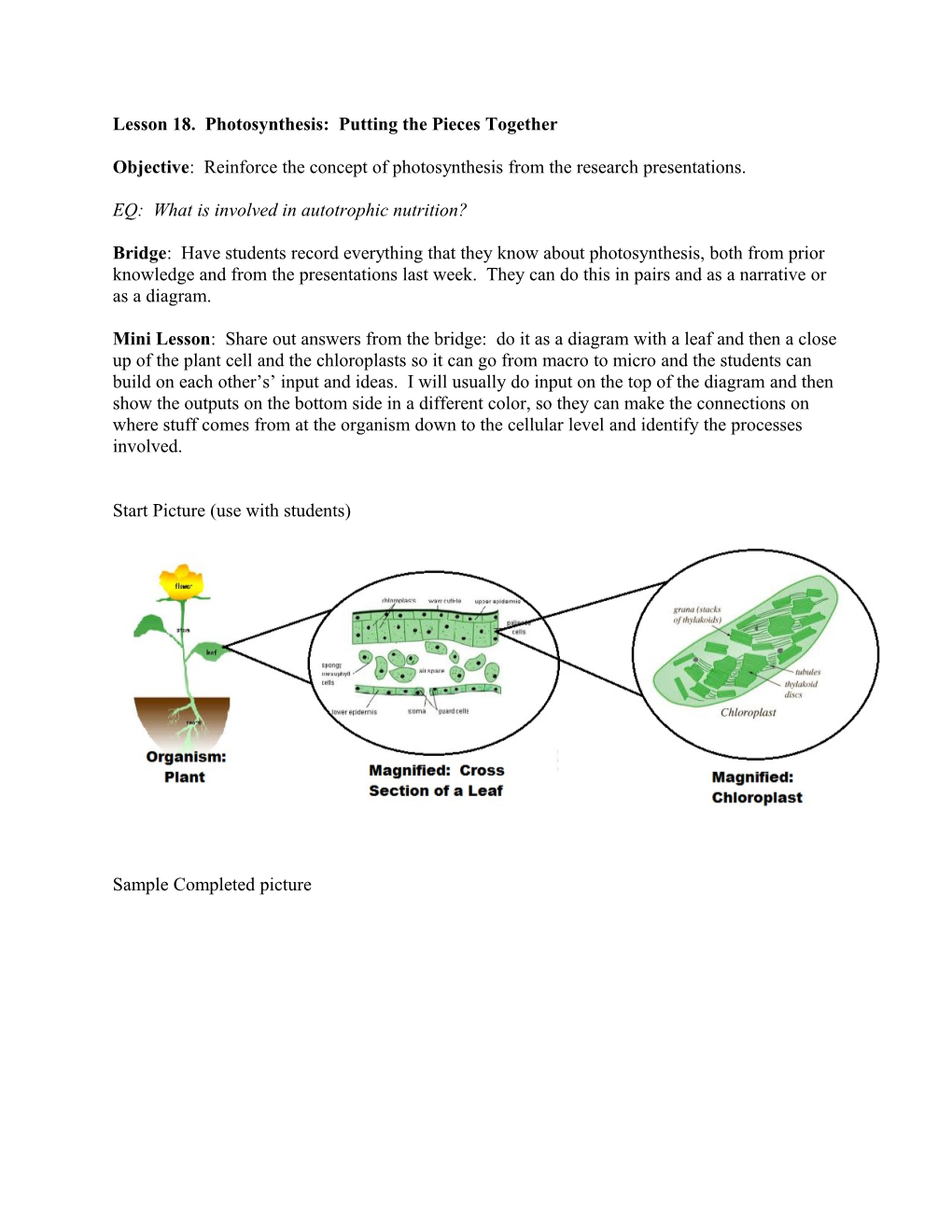
Mini Lesson: Share out answers from the bridge: do it as a diagram with a leaf and then a close up of the plant cell and the chloroplasts so it can go from macro to micro and the students can build on each other’s’ input and ideas. I will usually do input on the top of the diagram and then show the outputs on the bottom side in a different color, so they can make the connections on where stuff comes from at the organism down to the cellular level and identify the processes involved.
Start Picture (use with students)
Sample Completed picture Work Period: Students will read the letters or journal entries from a selection of the scientists that discovered the pieces to put together the process of photosynthesis. As they read, have students reflect on the behaviors that these scientists used to discover each piece of the photosynthesis puzzle and relate it back to their completed diagram. Remind them that their purpose is not just reading, but isolating the way in which these scientists worked, how they built off each other, and used evidence to support their hypotheses, including poor science practices.
I usually do this at stations, just to get them up and moving around, providing a few copies of each letter or article and have them work in pairs so they can discuss the readings as they go through.
Summary: Answer the EQ
Closing: Write the process of photosynthesis above from your diagram as a chemical formula, since that is in essence what it is.
SPED and ELL modifications: 1. Prerecord all the letter s for the work period so students who are poor readers can listen to them as many times as needed. 2. Have a visual model of the chemical process of photosynthesis for students to look at 3. Show a video of the process of photosynthesis at one of the stations using a video: This website has both a visual model of the chemical formula and lower and higher level videos explaining photosynthesis: http://www.chemicalformula.org/photosynthesis Apps and Internet Activities:
1. There are several apps that offer flashcards and videos on photosynthetic processes and plants Botany App: 101 concepts biology app: VCEll Principles of Life Flashcards – chapter 6
Independent Practice: Regents Questions on Photosynthesis
London, 1. July 1772 Benjamin Franklin, I am fully convinced that the air, which is made harmful by our breathing out, can be restored through plants. I have gathered used up air in a container and sealed this container hermetically. Seven days later, I placed a mouse into this container. In another container with the same used up air, I placed a plant. Seven days later I placed a mouse in the same container where the plant was. The mouse which was in the container without a plant died after 5 seconds. The mouse placed in the container with a plant, lived happily in the container for many minutes. Then I took the mouse out and placed it in the other container without any plants in. This poor mouse, which did so well in the container with the plant in, had to be taken out and resuscitated after spending as little as two seconds in the container without plants. This experiment shows us that plants can give the air its freshness back. Kind regards
Joseph Priestley
Journal Entry from Flemish physicist, Jan Baptista van Helmont Date of Experiment Unknown (sometime in 1642) Journal Entry published in 1648
But I have learned by this handicraft-operation that all Vegetables do immediately, and materially proceed out of the Element of water onely. For I took an Earthen vessel, in which I put 200 pounds of Earth that had been dried in a Furnace, which I moystened with Rainwater, and I implanted therein the Trunk or Stem of a Willow Tree, weighing five pounds; and at length, five years being finished, the Tree sprung from thence, did weigh 169 pounds, and about three ounces: But I moystened the Earthen Vessel with Rain-water, or distilled water (alwayes when there was need) and it was large, and implanted into the Earth, and least the dust that flew about should be co-mingled with the Earth, I covered the lip or mouth of the Vessel with an Iron-Plate covered with Tin, and easily passable with many holes. I computed not the weight of the leaves that fell off in the four Autumnes. At length, I again dried the Earth of the Vessell, and there were found the same two hundred pounds, wanting about two ounces. Therefore 164 pounds of Wood, Barks, and Roots, arose out of water onely.
Journal Entry, 1699 John Woodward In response to the experimentation of Helmont
I must confess I cannot see how this experiment can ever be made with the nicety and justness that is required, in order to build upon it so much as these gentlemen do. 'Tis hard to weigh Earth in that quantity, or plants of the size of those they mention, with any great exactness: or to bake the Earth with that accuracy, as to reduce it twice to the same dryness.
What about the 2 ounces of soil that were missing from his earthen pot? That must have gone somewhere. Even in van Helmont’s previous observations he noted that upon burning a dried plant that there is ash remaining in the end thus one could conceive that the contents of this ash must have come from something, not from nothing. Hence I will endeavor to fill in some of these missing facts.
Results of Woodward’s experiment, published in 1699:
Table 1. Effect of water source on spearmint (Mentha spicata) growth and transpiration in water culture (Woodward, 1699).* Modified from original notes for organizational purposes. Water source % fresh wt. gain Transpiration Ratio** plain rep. 1 100 111 plain rep. 2 126 95 plus soil rep. 1 222 64 plus soil rep. 2 309 53 distilled 36 215
*Glass containers were covered by parchment to prevent evaporation. The stem was inserted through a hole in the parchment. Plants were grown for 56 days in a windowsill in June and July 1692. **Grams of water lost divided by grams of fresh weight gained by plant.
From this I can conclude that plants that have been grown in soil with waters other than those that have been distilled and thus the plants must need something from the soil other than water. This accounts for the 2 ounces missing from Helmont’s experiment.
It is my opinion that those conclusions brought forth by van Helmont represent only a partial truth. He has ignored science in favor of accepting his beliefs and ignoring pressing facts in regards to fact, including previous experimental data that has hence been published in his works on gasses. He has also overlooked the works of those in the agriculture industry using water only in which to grow their livings.
There is still more work to be done.
“I took some pains to disclose the cause of these bubbles, which, I think, are of more importance than Mr. Bonnet at that time imagined them to be” ~Jan Ingen- Housz
First Set of Experiments Summary:
Plants were placed in clear, closed containers, under different kinds of water in the presence of light:
Fresh water Water from a river Rain water Boiling water Stagnant water Distilled water
Ingen-Housz made observations of the bubbles that were forming on the undersides of the leaves.
Conclusion:
“The most part of leaves gather these bubbles upon their surface, when plunged in any water in the sun- shine or by day-time in the open air; but infinitely more in fresh pump water than in any other. In clear river water they appear later, less in number and in size; less so in rain water, and the least of all in boiled water, in stagnating, and in distilled water. “ Second Set of Experiments Summary:
Same set up was used, but this time he varied the temperature of the water Water warmed by the sun Water at room temperature out of the sun’s rays Water that had been near freezing
And made observations of the bubbles that were forming on the undersides of the leaves
Conclusion: “They [the bubbles] are not produced by the warmth of the sun rarifying the air adhering to the leaves; for many kinds of leaves produce them almost as soon as plunged under water, though the water be very cold, and the leaves warm from the sun-shine be plunged in it.”
These observations led Ingen-Housz to investigate the fact that indeed it is the sun’s light and not the warmth of the sun that causes the air to be produced.
Conclusion: I placed some leaves in pump water, inverted the jar, and kept it near the fire as was required to received [sic] a moderate warmth, near as much as a similar jar, filled with leaves of the same plant, and placed in the open air, at the same time received from the sun. The result was, that the air obtained by the fire was very bad, and that obtained in the sun was dephlogisticated air. A jar full of walnut tree leaves was placed under the shade of other plants, and near a wall, so that no rays of the sun could reach it. It stood there the whole day, so that the water in the jar had received there about the same degree of warmth as the surrounding air (the thermometer being then at 76°); the air obtained was worse than common air, whereas the air obtained from other jars kept in the sun-shine during such a little time that the water had by no means received a degree of warmth approaching that of the atmosphere, was fine dephlogisticated air. Third Set of Experiments:
Same set up was used, but this time he repeated both sets of experiments in the dark and made observations of the bubbles that were forming on the undersides of the leaves
Conclusion: “They [the bubbles] do not appear after sun-set, at least not in any considerable number; but those that already exist do not shrink in or disappear by the cold of the night.”
This led the overall conclusion for Ingen-Housz that plants indeed needed light in order to survive and the light was somehow responsible for whatever was in the bubbles he observed in the water.
He then writes to his friend and colleague, Joseph Priestly, about his discovery. Dr. Priestly,
I have made advancements in your study of plants. It is with pride that I acknowledge your success in discovering the clarifying properties of plants in regards to the airs around us. Your work inspired me to focus some of my studies on those airs and the sunlight that plants appear to need. I have recently found, thus, that your airs can be quantified by submergence of those plants under water. Bubbles are formed in sun-light hours and not in sun-set conditions and the types of waters are as important as the types of airs.
This discovery has thus led me to ask the question “will the light shone down upon the plants make changes to the quality of airs presented henceforth by my dear friend and colleague Joseph Priestly and his laboratory rodents?” It is this question that has led to the composition of this letter and communication with you: I would like to repeat your experiment with the mice, but focus on the light available to the plants as the driving force of such discovery. I await your return communicae in this matter and I will be travelling henceforth to the same cottage laboratory where you isolated your very important life air.
Your Humble Admirer,
Jan Ingen-Housz
Upon receipt of the response from Joseph Priestly, Ingen-Housz then proceeded to test how those bubbles that arose from the plants affected organisms in surrounding environments. He set the experiment up using the same equipment in the same location that Priestly did.
First, he put a plant and a candle into a transparent closed space. He allowed the system to stand in sunlight for two or three days. This assured that the air inside was pure enough to support a candle flame. But he did not light the candle. Instead, he covered the closed space with a black cloth and let it remain covered for several days. When he tried to light the candle it would not light.
Conclusion:
“This observation leads me to believe that somehow the plant must act in darkness like an animal. It must have breathed, fouling the air, and therefore the candle will not light without the presence of Priestly’s air. In order to purify the air plants need light.” Next, he repeated the mouse experiment in much the same way. He placed 2 plants in closed, transparent containers and let them sit for 2-3 days. He placed the mice inside to find that they lasted 5 minutes before they started to show signs of struggling. He then left those plants, one in sun and one he covered with a dark cloth again and left them for another 2-3 days. After the 3 days, he placed the mice in the containers to find the one in the light allowed to mouse to survive while the one in the dark started to kill the mouse immediately.
Conclusion: “To build on Dr. Priestly’s experiment, plants are not only purifying agents to the airs around us, but light is the catalyst by which the air is purified. Therefore, if there is no light available to the plants, then the air remains foul, perhaps even more foul than with just a mouse.”
Conclusion are all quotes (or paraphrased from the quotes for interpretation reasons) taken from An Essay on the Food of Plants and the Renovation of Soils by John Ingen- Housz